-
PDF
- Split View
-
Views
-
Cite
Cite
Natsuki Tanaka, Takashi Fujiwara, Rie Tomioka, Ute Krämer, Miki Kawachi, Masayoshi Maeshima, Characterization of the Histidine-Rich Loop of Arabidopsis Vacuolar Membrane Zinc Transporter AtMTP1 as a Sensor of Zinc Level in the Cytosol, Plant and Cell Physiology, Volume 56, Issue 3, March 2015, Pages 510–519, https://doi.org/10.1093/pcp/pcu194
Close - Share Icon Share
Abstract
The vacuolar Zn2+/H+ antiporter of Arabidopsis thaliana, AtMTP1, has a long cytosolic histidine-rich loop. A mutated AtMTP1 in which the first half of the loop (His-half) was deleted exhibited a 11-fold higher transport velocity in yeast cells. Transgenic lines overexpressing the His-half-deleted AtMTP1 in the loss-of-function mutant were evaluated for growth and metal content in the presence of various zinc concentrations. These overexpressing lines (35S-AtMTP1 and 35S-His-half lines) showed high tolerance to excess concentrations of zinc at 150 µM, as did the wild type, compared with the loss-of-function line. The His-half AtMTP1 transported cobalt in a heterologous expression assay in yeast, but the cumulative amount of cobalt in 35S-His-half plants was not increased. Moreover, the accumulation of calcium and iron was not changed in plants. Under zinc-deficient conditions, growth of 35S-His-half lines was markedly suppressed. Under the same conditions, the 35S-His-half lines accumulated larger amounts of zinc in roots and smaller amounts of zinc in shoots compared with the other lines, suggesting an abnormal accumulation of zinc in the roots of 35S-His-half lines. As a result, the shoots may exhibit zinc deficiency. Taken together, these results suggest that the His-loop acts as a sensor of cytosolic zinc to maintain an essential level in the cytosol and that the dysfunction of the loop results in an uncontrolled accumulation of zinc in the vacuoles of root cells.
Introduction
Zinc (Zn) is an essential micronutrient that functions as a cofactor for many enzymes and proteins, including DNA polymerase, Cu/Zn superoxide dismutase and Zn-finger proteins (Greger 2004, Krämer 2005, Clemens 2010, Krämer 2010). It also leads to toxicity in excess concentrations (Rogers et al. 2000, Kobae et al. 2004, Fukao et al. 2011, Sinclair and Krämer 2012). Zn deficiency causes growth suppression of whole plants and chlorosis of leaves, whereas excess amounts of Zn2+ suppress root elongation. Zn in excess amounts enhances the generation of reactive oxygen species in the cell proliferation zone of roots and suppresses growth (Kawachi et al. 2009). Under excess Zn conditions, photosynthesis activity decreases caused by disorder of the Fe homeostasis (Sinclair and Krämer 2012). Zn homeostasis in cells, particularly cytosolic Zn2+ concentrations, is essential for the normal growth of plants. Excess Zn2+ is exported from the cytosol to the extracellular space and vacuoles via plasma membrane Zn transporters and vacuolar membrane Zn transporters, respectively. Arabidopsis thaliana has metal tolerance proteins (MTPs), zinc-regulated transporters/iron-regulated transporter-like proteins (ZIPs) and heavy-metal ATPases (HMAs) (P1B subgroup of P-type ATPases), which differ in structure and transport mechanism (MacDiarmid et al. 2002, Krämer et al. 2007, Hanikenne et al. 2008, Morel et al. 2009, Kawachi et al. 2011, Shanmugam et al. 2013). ZIPs are transporters for translocation of Zn into the cytosol at the plasma membrane and membranes of intracellular organelles (Lin et al. 2010). MTP is a subfamily of the cation diffusion facilitator (CDF) family and uses a pH gradient across the membrane for active Zn transport. HMA actively transports Zn2+ using energy provided by ATP hydrolysis. Most organisms regulate the gene expression of these Zn transporters and channels by modulating Zn-binding transcription factors in response to Zn deficiency and toxicity (Eide 2009).
Among the 12 members of the MTP subfamily (Blaudez et al. 2003), AtMTP1 functions as an active transporter in the vacuolar membrane to sequester excess Zn2+ into the vacuole and maintain a low cytosolic Zn2+ concentration (Desbrosses-Fonrouge et al. 2004, Kawachi et al. 2009, Krämer et al. 2010). AtMTP1 is essential for basal tolerance of excess amounts of Zn2+ by A. thaliana, as demonstrated by the observation that the loss-of-function mutant mtp1 is phenotypically sensitive to excess Zn2+ (Kobae et al. 2004, Martinoia et al. 2007). The hyperaccumulator Arabidopsis halleri, which has up to five copies of MTP1 and a total MTP1 protein level higher than that of A. thaliana, shows extremely high tolerance to excess Zn (Becher et al. 2004, Dräger et al. 2004, Shahzad et al. 2010). The MTP subfamily members have six transmembrane helices and a relatively large and conserved C-terminal domain that binds Zn2+ in the cytosol. In addition to this common structure (Lu et al. 2009), AtMTP1 has a His-rich loop (His-loop), which faces the cytosol, between the fourth and fifth transmembrane helices (Kawachi et al. 2008). Other MTPs, such as A. halleri MTP1, Noccaea goesingense MTP1t1 (formerly TgMTP1t1), Populus MTP1, Saccharomyces cerevisiae Cot1 and Homo sapiens ZnT1, also have a His-loop, although the number of histidine residues and the length of the loop are different (Kawachi et al. 2008, Krämer 2010). Metal transporters with a His-loop were found in all plant species whose genomes have been sequenced. However, the His-loop is absent in Escherichia coli YiiP, which is a member of the CDF family and has been well characterized in terms of 3D structure and catalytic mechanisms (Lu and Fu 2007, Lu et al. 2009). Information on the higher order structure and physiological role of the His-loop is limited at present. The mutated AtMTP1 that lacked the first half of the His-loop (His-half) conferred an enhanced Zn2+ transport activity (Kawachi et al. 2008). The His-loop was recently found to comprise partly an intrinsically disordered structure binding four Zn2+ per molecule, with the Zn2+ binding inducing a structural change in the His-loop (Tanaka et al. 2013).
We investigated the physiological role of the His-loop in the ion selectivity and ion transport activity of AtMTP1 in growing plants. By heterologous expression of the mutated AtMTP1 in yeast cells, we found earlier that deletion of the whole sequence of the His-loop resulted in inactivation of AtMTP1. In contrast, the His-half AtMTP1, which lacks the first half with 32 residues (position 185–216 of AtMTP1) of the His-loop, retained the Zn2+ transport activity (Kawachi et al. 2008). The Vmax value of His-half AtMTP1 was 11-fold that of the normal AtMTP1 and the Km for Zn2+ was twice that of AtMTP1. The His-half AtMTP1 also transported Co2+ in yeast cells. Thus, the His-loop of AtMTP1 is not essential for Zn2+ transport and was proposed to act as a buffering pocket of Zn2+, a sensor of the Zn level and a Zn2+ selection domain (Kawachi et al. 2008, Kawachi et al. 2012). To evaluate the effect of the enhanced activity, lack of Zn sensor and altered ion selectivity of the His-half AtMTP1 on the growth of plants, we expressed the His-half AtMTP1 in mtp1-1 homozygous mutant plants and measured their growth, tolerance to Zn excess and deficiency, and amount of Zn incorporated in tissues. We obtained key information on the physiological role of the His-loop. Together with the results, we discuss the biochemical and physiological significance of the His-loop.
Results
Growth of 35S-His-half lines in excess Zn and Co
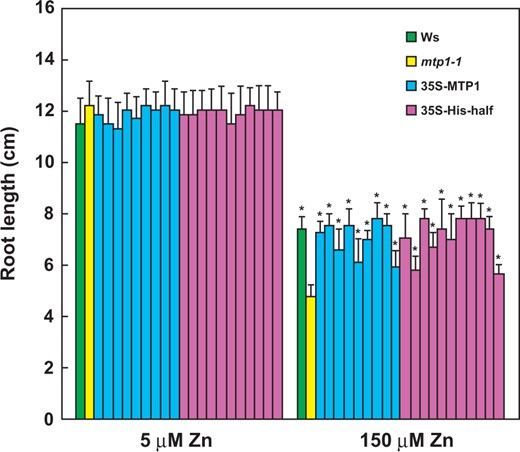
The sensitivity of the loss-of-function mutant mtp1-1 to excess Zn2+ indicates that AtMTP1 is important in Zn homeostasis, particularly the detoxification of excess cytosolic Zn into the vacuole (Kobae et al. 2004, Kawachi et al. 2009). A mutant AtMTP1 lacking the first half of the His-loop (32 residues; His-half; refer to membrane topology models in Supplementary Fig. S1) enhanced Zn2+ transport activity when expressed in S. cerevisiae (Kawachi et al. 2008). We accordingly investigated whether plants expressing the His-half AtMTP1 (35S-His-half lines) are more tolerant to excess Zn2+ than wild-type plants.
35S-His-half lines grew as well as the wild type (Ws) and 35S-MTP1 under Zn excess conditions. Seedlings of Ws, mtp1-1, 35S-MTP1 and 35S-His-half lines were grown in modified Hoagland medium in which the Zn concentration was modified to 150 µM or 5 µM (normal). Independent lines of 35S-MTP1 (nine lines) and 35S-His-half (11 lines) were examined. After 14 d, the root length was measured. For each line, 21 plants were independently analyzed. Values are expressed as mean ± SD. Significant differences from mtp1-1 (a loss-of-function line) are indicated by asterisks (*P < 0.01).
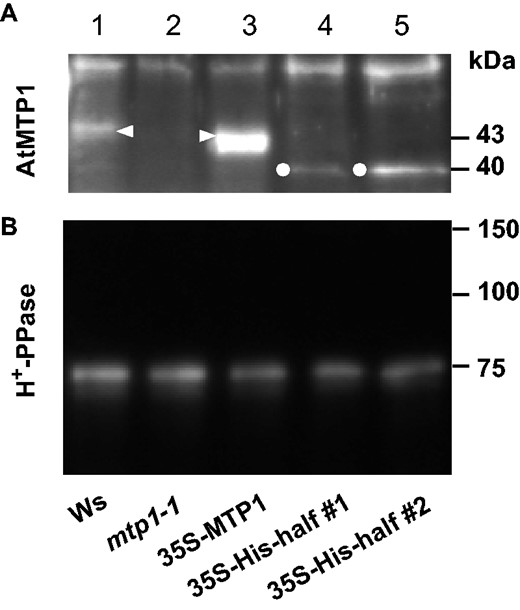
Immunoblotting of AtMTP1 and H+-PPase. Vacuolar membranes were prepared from 14-day-old plantlets of Ws and transgenic lines. Aliquots corresponding to 160 mg (A) and 40 mg (B) of fresh weight were subjected to SDS–PAGE and subsequent immunoblotting with anti-AtMTP1 (A) and anti-H+-PPase (B) antibodies.
As shown in Fig. 2A, the protein level of the His-half AtMTP1 was slightly different between two His-half lines, in which 35S-His-half #2 was a line with higher expression. The difference in the protein level was partially correlated with recovery of root length in the His-half-2 line under excess Zn conditions at 200 and 250 µM but not at 5 or 100 µM (Supplementary Fig. S2).
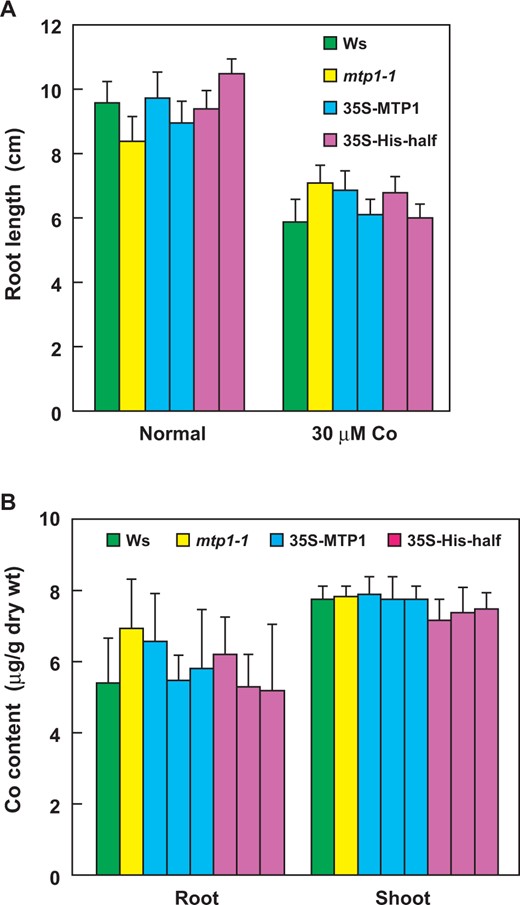
35S-His-half lines grew and accumulated Co as well as Ws in the medium supplemented with Co. Seedlings of Ws, mtp1-1, 35S-MTP1 and 35S-His-half lines were grown in modified Hoagland medium supplemented with CoSO4 at 0 or 30 µM (A) or in MS medium supplemented with CoSO4 at 3 µM (B). (A) The root length of 14-day-old seedlings was measured. Values are expressed as mean ± SD; n = 21. (B) The content of Co in 21-day-old seedlings was measured by ICP-AES. Values are expressed as mean ± SD; n = 4.
Growth suppression in 35S-His-half lines under Zn-deficient conditions
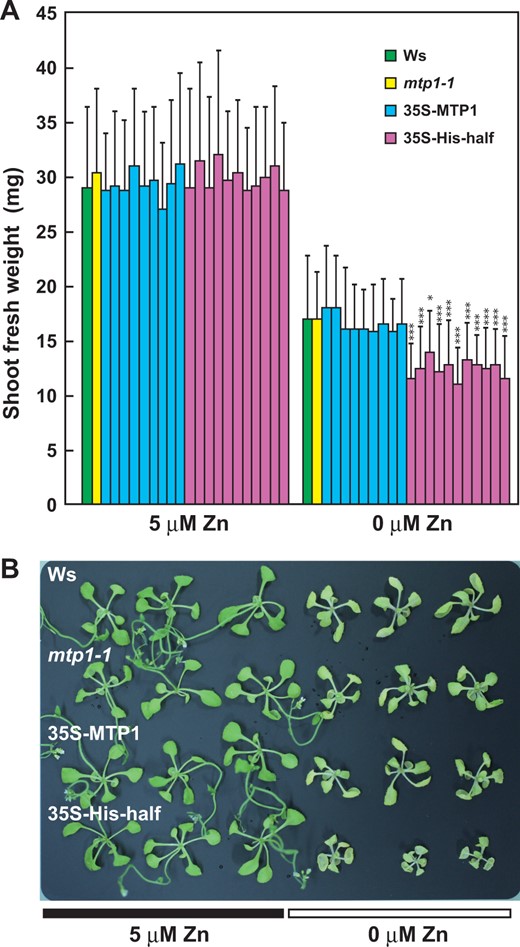
AtMTP1 actively transports Zn2+ into vacuoles by acting as a Zn2+/H+ exchanger (Kobae et al. 2004, Kawachi et al. 2008) and is highly expressed in the apex of primary and lateral roots (Desbrosses-Fonrouge et al. 2005, Kawachi et al. 2009). Thus, Zn2+ may be accumulated at a high level in roots as compared with shoots. In contrast to AtMTP1, the His-half AtMTP1 does not suppress the transport activity under Zn-deficient conditions. Therefore, shoots of the 35S-His-half lines cannot receive sufficient Zn2+ from the roots when grown under Zn-deficient conditions. We examined this hypothesis by testing the tolerance of the seedlings of Ws and mutant lines under Zn-deficient conditions. To reduce the Zn concentration, we added a membrane-permeable, Zn2+ chelator N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN) (Arslan et al. 1985, Shumaker et al. 1998) to modified Hoagland medium that was not supplemented with Zn2+. Seven-week-old seedlings showed symptoms of critical Zn deficiency, such as chlorosis and growth delay (Supplementary Fig. S4). Older leaves of Ws, mtp1-1 and 35S-MTP1 lines were light yellow and their younger leaves remained light green. The 35S-His-half lines suffered more severe damage and their younger and older leaves became light yellow. Furthermore, the growth of 35S-His-half lines was severely suppressed, and the fresh weight of 35S-His-half shoots tends to be lower than those of Ws, mtp1-1 and 35S-MTP1 lines (Supplementary Fig. S4).
To confirm these results, we established Zn deficiency without the use of excess chelator. We quantified the Zn content in agar products produced by different manufacturers by ICP-AES and used the one lowest in Zn content for seedling cultivation. Among type M agar (Sigma-Aldrich; 1.01 µg g–1 dry matter), Ina agar (type BA-10, Funakoshi; 0.200 µg g–1), purified agar (Nacalai Tesque; 0.530 µg g–1) and gellan gum (Wako Pure Chemical; 1.35 µg g–1), the Ina agar showed the lowest value of Zn content and was used in the experiments. Agar and related agents were dissolved in ultra-pure water to eliminate contamination of the agar by water.
35S-His-half lines grew poorly under Zn-deficient conditions. Plants of Ws, mtp1-1, 35S-MTP1 and 35S-His-half lines were grown in modified Hoagland medium lacking Zn (0 µM Zn) or with 5 µM Zn (normal conditions). Independent lines of 35S-MTP1 (nine lines) and 35S-His-half (11 lines) were examined. After 21 d, the shoots were weighed. Values are expressed as mean ± SD; n = 36. Significant differences from WS are indicated by asterisks (*P < 0.05; ***P < 0.005). (B) Three shoots each from Ws, mtp1-1, 35S-MTP1 and 35S-His-half lines grown under normal (5 µM Zn) and Zn-deficient conditions (0 µM Zn) for 27 d were photographed.
In this experiment, damage of the leaves by Zn deficiency was mild compared with that in the medium containing TPEN (Supplementary Fig. S4), because leaf color remained green even after 27 d (Fig. 4). We concluded that this experimental condition was suitable for the evaluation of Zn deficiency in living plants and used it in subsequent experiments.
Shoot–root ratio of Zn content was reduced in 35S-His-half lines
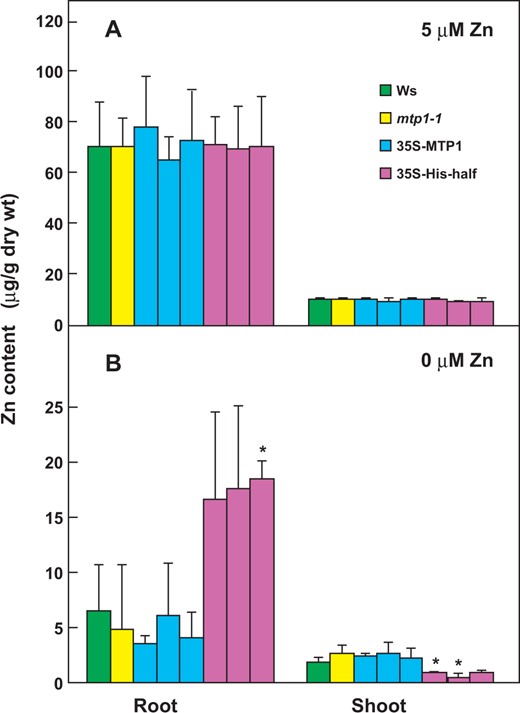
35S-His-half lines accumulated higher amounts of Zn in roots than the wild type even under Zn-deficient conditions. Seedlings of Ws, mtp1-1, 35S-MTP1 and 35S-His-half lines were grown in modified Hoagland medium lacking Zn (0 μM Zn) or with 5 μM Zn. After 21 d, the contents of Zn in shoots and roots were measured by ICP-AES. Values are expressed as mean ± SD; n ≥ 4. Significant differences from Ws are indicated by asterisks (*P < 0.05).
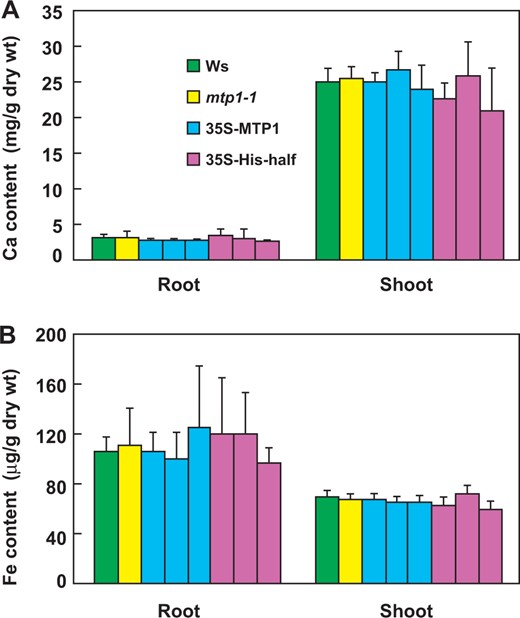
The 35S-His-half mutant accumulated the same amounts of Ca and Fe as the wild type under Zn-deficient conditions. Seedlings of Ws, mtp1-1, 35S-MTP1 and 35S-His-half lines were grown in modified Hoagland medium lacking Zn. The contents of Ca (A) and Fe (B) in shoots and roots of 21-day-old plants were measured by ICP-AES. Values are expressed as the mean ± SD; n ≥ 4.
Chloroplast number and Chl content in 35S-His-half lines were reduced under Zn-deficient conditions
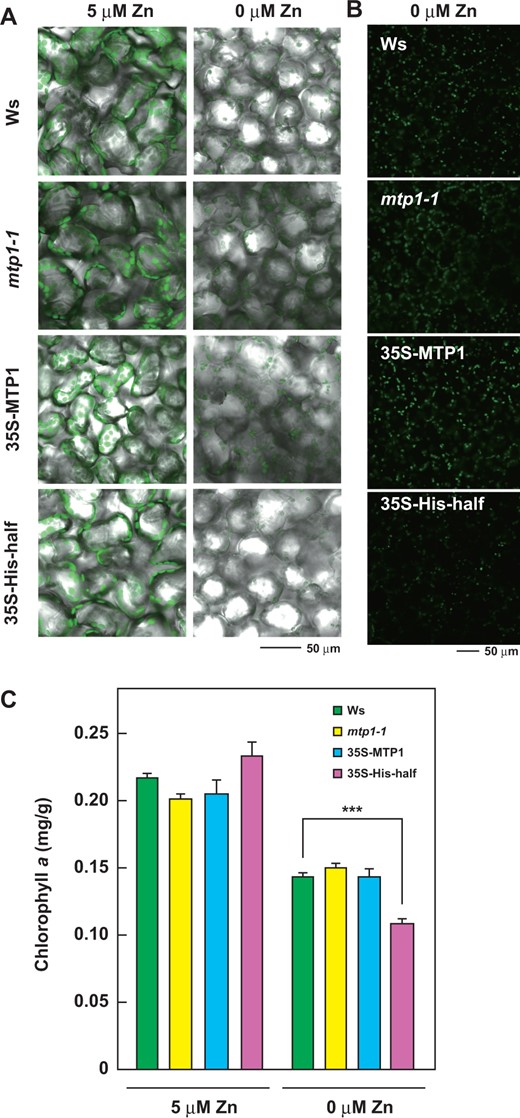
Chloroplasts and Chl content in the leaves of 35S-His-half lines were lower than those in other lines. Plants including Ws, mtp1-1, 35S-MTP1 and 35S-His-half lines were grown for 27 d in modified Hoagland medium lacking Zn (0 µM Zn) or with 5 µM Zn. The second layers of leaf epidermis were observed using a confocal laser scanning microscope. (A) Merged images of autofluorescence (green) of chloroplasts and differential interference contrast images. (B) Images of autofluorescence of chloroplasts of four lines. (C) Chl a content in leaves of Ws, mtp1-1, 35S-MTP1 and 35S-His-half lines grown under normal (5 µM Zn) and Zn-deficient (0 µM) conditions.
Discussion
AtMTP1 functions as a scavenger of excess Zn2+ in the cytosol by translocating Zn2+ into vacuoles. This function is evident from the phenotype property of mtp1, which is extremely sensitive to excess Zn2+ in culture medium (Kobae et al. 2004, Desbrosses-Fonrouge et al. 2005, Krämer 2010). Furthermore, mtp1-1 lines accumulated less Zn2+ in their roots and shoots than the wild type (Kawachi et al. 2009). We focused attention on the His-loop, which is distinctive for a subgroup of so-called Zn-CDF proteins from plants (Montanini et al. 2007). The His-half AtMTP1 conferred an enhanced activity and transported Co2+ when expressed in yeast cells (Kawachi et al. 2008). The His-loop of AtMTP1 is not essential for Zn2+ transport and was proposed to play the roles of a buffering pocket for Zn2+ and a sensor of the Zn2+ level, and to be involved in ion selectivity (Kawachi et al. 2008, Kawachi et al. 2012). This hypothesis was tested by characterization of transgenic lines expressing the His-half AtMTP1.
First, it should be noted that the His-half AtMTP1 is functional in transgenic plants, given that the His-half AtMTP1 complemented the lack of normal AtMTP1. The 35S-His-half lines recovered their tolerance to excess Zn, as shown by root elongation (Fig. 1). Furthermore, the His-half AtMTP1 protein was clearly detected in an immunoblot (Fig. 2). The His-half AtMTP1 efficiently transports excess cytosolic Zn2+ into vacuoles. We accordingly inferred that 35S-His-half lines show higher tolerance to excess Zn than Ws. However, the root length of 35S-His-half lines was the same as that of Ws under Zn excess conditions. We may suggest two possible explanations for this phenomenon: (i) the cytosol Zn2+ concentrations were too high to be removed by the His-half AtMTP1, although the mutated AtMTP1 actively transported Zn2+ into vacuoles; or (ii) the vacuolar Zn2+ concentration and/or a gradient of Zn2+ concentration across the vacuolar membrane was too high for active Zn2+ transport by the His-half AtMTP1. These explanations are supported by the observation that 35S-MTP1 also showed no extra tolerance to excess Zn2+ (Fig. 1).
The expression of the His-half AtMTP1, which was shown to transport Co2+ (Kawachi et al. 2008), did not change the tolerance to Co or content of Co as in the 35S-MTP1 lines (Fig. 3). A. thaliana probably has other Co-selective transporter(s) in the vacuolar membrane, and the contribution of the His-half AtMTP1 is negligible in plants.
Secondly, we discuss the physiological role of the His-loop of AtMTP1 in plants in view of the results of growth and Zn content in tissues under Zn-deficient conditions. Growth suppression of plants and a decrease in Chl a content are typical symptoms of Zn deficiency. These symptoms were observed with the medium containing a low concentration of TPEN, and were most severe in the 35S-His-half lines (Supplementary Fig. S4). However, cultivation in the medium containing TPEN seemed to damage plant tissues, probably due to removal of not only free Zn2+ but also other divalent metal ions from tissues, given that TPEN has relatively high affinity for Zn2+, Fe2+ and Mn2+ (Arslan et al. 1985, Thamatrakoln and Hildebrand 2008). In the other experiments, the agar with the lowest content of Zn and highly purified water were used to evaluate the effects of Zn deficiency. This cultivation medium may be suitable judging from the low Zn content in plants under the conditions mentioned below. Plants showed reductions of shoot fresh weight due to Zn deficiency (Fig. 4). In 35S-His-half lines, additional significant suppression was observed in shoot fresh weight. Suppression of growth in 35S-His-half shoots may be caused by the shortage of Zn in shoots.
The Zn contents in roots and shoots of 21-day-old plants cultivated under normal conditions were approximately 70 and 10 µg g DW–1, respectively (Fig. 5). This imbalance of Zn between roots and shoots, representing Zn immobility in plants, is consistent with previous reports (Talke et al. 2006, Kawachi et al. 2009, Fukao et al. 2011). Zn may be predominantly sequestered in root cells, particularly in vacuoles, and transferred to shoots as needed. Under Zn-sufficient conditions, root cells absorb sufficient Zn2+ and the excess Zn2+ was transported into the vacuoles in roots via AtMTP1 and AtMTP3. Part of the Zn2+ in the root cells was translocated to shoots (Fig. 5A). Under Zn-sufficient conditions, there may be no difference in the Zn contents between Ws and 35S-His-half lines because normal AtMTP1 may express its full activity at high Zn2+ concentrations.
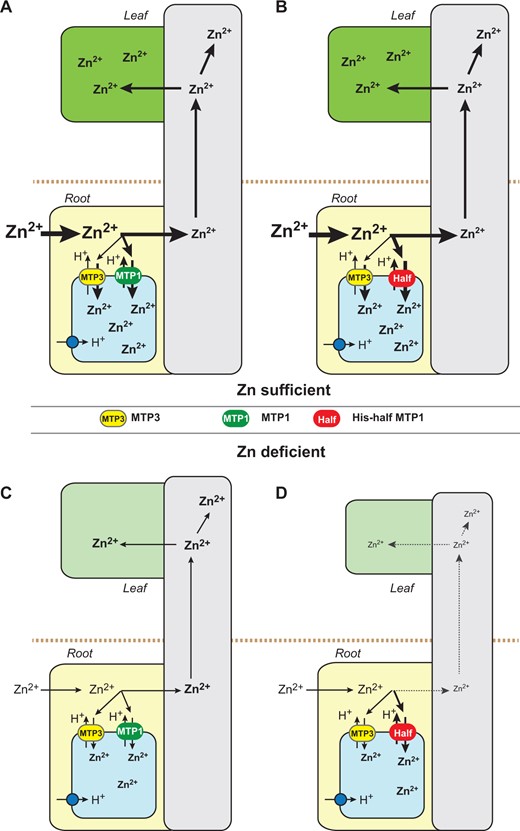
Schematic model of the physiological effect of the AtMTP1 His-loop in plants under Zn-deficient conditions. Flows of Zn ions in plants expressing normal AtMTP1 (A and C) and the His-half AtMTP1 with high transport activity (B and D) under normal (A and B) and Zn-deficient conditions (C and D) are shown. AtMTP1 and the His-half AtMTP1 function as Zn2+/H+ exchangers with the help of proton pumps (blue circles). In the vacuolar membrane, another Zn2+/H+ exchanger, AtMTP3 (yellow ovals), functions in sequestration of Zn into vacuoles. Part of Zn taken from the culture medium or soils by roots is compartmentalized into the vacuoles in root cells by normal AtMTP1 (green ovals) or the His-half AtMTP1 (red ovals), and the remainder of Zn is transported to the vascular tissues and then transferred to shoots. The thickness of arrows corresponds to the flow amount of Zn in tissues. Zn contents in roots and tissues are shown according to the results in Fig. 5. The letter size of Zn2+ indicates the difference of Zn2+ levels.
At present, the molecular mechanism of the suppression of Zn transport activity specifically under low-Zn conditions in contrast to high-Zn conditions remains to be resolved. Several amino acid residues in the His-loop are involved in Zn transport and ion selectivity (Podar et al. 2012). The His-loop partly comprises a polyproline type II structure and binds four Zn2+ per molecule (Tanaka et al. 2013). The conformational change of the His-loop by Zn2+ binding to the loop has also been shown by circular dichroism spectroscopy (Tanaka et al. 2013). Kawachi et al. (2012) emphasized the functional importance of Asn258 for regulation of Zn transport activity. This Asn258 is located at the cytosolic side of the fifth transmembrane domain and linked to the His-loop, as inferred from the 3D structure of E. coli YiiP (Lu et al. 2009). It is accordingly considered that the His-loop is not occupied by Zn under low-Zn conditions and that the entrance to the Zn transport pore is obstructed by Asn258. Under high-Zn conditions, the His-loop changes its conformation and opens the translocation pore by changing the conformational position of Asn258 at the cytosolic side. This hypothesis should be tested by determination of the complete structures of AtMTP1 with or without Zn2+ bound to the His-loop.
In conclusion, this study revealed that the His-loop is involved in suppression of the Zn transport activity of AtMTP1 under Zn-deficient conditions. This suppression may be essential for plant growth under Zn-deficient conditions. MTP1 and related transporters in the CDF family in plants and various organisms have the His-loop, and this loop may, like AtMTP1, play a key role in Zn2+ homeostasis in the cytosol.
Materials and Methods
Plant materials and growth conditions
Arabidopsis thaliana strain Ws was used as the wild type. A homozygous loss-of-function mutant line mtp1-1 was prepared as described previously (Kobae et al. 2004). Seeds were surface-sterilized and germinated on sterile gel plates containing 2.5 mM MES-KOH, pH 5.7, 1% (w/v) sucrose, 1.2% Ina agar (Funakoshi) and modified Hoagland medium (Haydon et al. 2012) for the preparation of Zn-deficient agar plates. The modified Hoagland medium contained 1.5 mM Ca(NO3)2, 0.28 mM KH2PO4, 0.75 mM MgSO4, 1.25 mM KNO3, 0.5 µM CuSO4, 5 µM ZnSO4, 5 µM MnSO4, 25 µM H3BO3, 0.1 µM Na2MoO4, 50 µM KCl, 5 µM Fe-HBED and 3 mM MES-KOH, pH 5.7. Standard agar plates contained Murashige and Skoog (MS) salt (Wako Pure Chemical), 2.5 mM MES-KOH, pH 5.7, 1% (w/v) sucrose and 1.2% Ina agar. ZnSO4 and CoSO4 were added to the medium at the indicated concentrations to evaluate the tolerance to excess Zn or Co. Seedlings were grown in the medium containing TPEN, a Zn chelator, to evaluate growth under Zn-deficient conditions (Thamatrakoln and Hildebrand 2008). Seedlings were grown at 22°C under long-day conditions (16 h light/8 h dark at 80–110 µmol m–2 s–1). In all experiments, the seeds were incubated at 4°C for 3 d in the dark before culture on medium.
Production of transgenic plants
cDNAs for an AtMTP1 (Kobae et al. 2004) and a Δ185–216 mutant AtMTP1 (Kawachi et al. 2008) in pKT10 vectors were prepared as described previously. The cDNAs for the pENTR entry vector were cloned into pGWB2 binary vector with the 35S promoter of Cauliflower mosaic virus, introduced into the Agrobacterium tumefaciens GV3101::PM90 strain and then used to transform mtp1-1 homozygous mutant plants by the floral dip method (Clough and Bent 1998). The resulting homozygous plants were used as mtp1-1:35S::AtMTP1 (35S-MTP1) and mtp1-1:35S::Δ185–216 AtMTP1 (35S-His-half) lines after selection with kanamycin and hygromycin. Plants of 9–11 independent T4 lines were used for physiological experiments.
Determination of mineral contents in plant tissues and agars
Arabidopsis thaliana seedlings were grown on agar plates, which were set vertically. After 21 d, shoots and roots were collected from seedlings, washed once in 10 mM EDTA and three times in ultra-pure water, and then dried at 70°C for 48 h. Dried tissues (40–100 mg) were digested with pure concentrated HNO3 for 25 min at 130°C in Teflon vessels (Ethos-1600). The mineral contents of the seedlings were then determined by ICP-AES (IRIS ICAP, Nippon Jarrell Ash).
The Zn content in agar products produced by different manufacturers was measured by ICP-AES. Agar products were from Sigma-Aldrich (type M), Funakoshi (Ina agar type BA-10) and Nacalai Tesque (agar purified). The Zn content of gellan gum (Wako Pure Chemical) was also determined.
Preparation of vacuolar membrane fraction and immunoblot analysis
Vacuolar membrane fractions were prepared from 14-day-old seedlings (n = 500) of Ws, mtp1-1, 35S-MTP1 and 35S-His-half plants grown in MS medium as described previously with some modifications (Maeshima and Yoshida 1989). Plants were homogenized in medium containing 0.25 M sorbitol, 50 mM Tris-acetate, pH 7.5, 1 mM EGTA, 20 µM p-(amidinophenyl)methanesulfonyl fluoride hydrochloride, 1% (w/v) polyvinylpyrrolidone and 2 mM dithiothreitol. The homogenates were centrifuged at 7,000×g at 4°C for 10 min, and the supernatants were centrifuged at 100,000×g at 4°C for 35 min. The pellets, which were crude membrane fractions, were suspended in the buffer containing 0.7 M sucrose, 20 mM Tris-acetate, pH 7.5, 1 mM EGTA and 2 mM MgCl2 using a Teflon homogenizer. The suspension was overlaid with 0.25 M sorbitol, 20 mM Tris-acetate (pH 7.5), 1 mM EGTA, 2 mM MgCl2 and 2 mM dithiothreitol. After centrifugation at 100,000×g for 30 min, the turbid interface portion was collected and diluted in 3 vols. of the homogenization medium. The suspension was then centrifuged at 100,000×g for 35 min, and the resulting precipitate (vacuolar membrane) was suspended in the buffer.
The proteins in the fractions were separated by SDS–PAGE and transferred to an Immobilon-P membrane (Millipore). After blocking with 5% defatted milk, the membrane filter was incubated with primary antibody and then with horseradish peroxidase-conjugated protein A (1 : 2,000) (GE Healthcare). Chemiluminescent reagents of ECL (GE Healthcare) were used for the detection of antigens. Antibodies specific to the N-terminal region of AtMTP1 (At2g46800, position 1–12, MESSSPHHSHIV) (Tanaka et al. 2013) and the substrate-binding region of H+-PPase (At1g15690, position 257–273, DLVGKIERNIPEDDPRN) (Segami et al. 2010) were used as anti-AtMTP1 antibodies (1 : 1,000 dilution) and anti-H+-PPase antibodies (1 : 4,000 dilution), respectively.
Microscopic observations
Images of chloroplast autofluorescence in cells of the second layer of leaf epidermis of a 27-day-old plant were obtained using an upright FV1000-D confocal laser scanning microscope (Olympus). The excitation wavelength and transmission range for emission were 473 nm and 630–730 nm, respectively.
Chl quantification
RNA extraction and real-time RT–PCR
Total RNA fractions were prepared from 22-day-old whole plants of Ws, mtp1-1, 35S-MTP1 and 35S-His-half lines using a QIA shredder and RNeasy Mini kit (Qiagen). RNA (0.5 µg) was converted into cDNA using a ReverTra Ace qPCR RT Master Mix with gDNA Remover (Toyobo). Real-time reverse transcription–PCR (RT–PCR) analysis was performed with a Thermal Cycler Dice™ Real Time System TP800 (TAKARA) using a THUNDERBIRD™ SYBR qPCR Mix (Toyobo). The primer sets used for the real-time RT–PCR were ATGGAGTCTTCAAGTCCCCAC (Fw) and CCGGAAGCATTCTTAGAATCTG (Rv) for AtMTP1, CTGATGTTGCAGCCTTTGCA (Fw) and TTTGCCTTCCATCCCGAA (Rv) for AtMTP3 and GTCGAGGTTTACGCATCCATG (Fw) and CTCGCCAAAAACCATTTCAG (Rv) for AtTIP4;1. Relative mRNA contents were normalized to the AtTIP4;1 transcript.
Funding
This work was supported by the Japan Society for the Promotion of Science (JSPS) [Grants-in-Aid Nos: 25650093 and 26252011]; the Steel Foundation for Environmental Protection Technology (2010-29); and the Japan Science and Technology Agency (JST) A-Step [AS242Z02907N] to M.M. N.T. received Research Fellowship for Young Scientists (doctoral course students) and Program for Leading Graduate School from the JSPS.
Acknowledgments
We are grateful to Dr. Yoichi Nakanishi and Dr Shoji Segami (Nagoya University) for their fruitful advice.
Disclosures
The authors have no conflicts of interest to declare.
Abbreviations
- CDF
cation diffusion facilitator
- His-loop
histidine-rich loop
- His-half
a deletion mutant lacking the first half of the histidine-rich loop
- H+-PPase; H+-pyrophosphatase; ICP-AES
inductively coupled plasma atomic emission spectroscopy
- MS
Murashige and Skoog
- MTP
metal tolerance protein
- TPEN
N, N, N′, N′-tetrakis(2-pyridylmethyl)ethylenediamine
References