-
PDF
- Split View
-
Views
-
Cite
Cite
Seong H. Lee, Janusz J. Zwiazek, Regulation of Aquaporin-Mediated Water Transport in Arabidopsis Roots Exposed to NaCl, Plant and Cell Physiology, Volume 56, Issue 4, April 2015, Pages 750–758, https://doi.org/10.1093/pcp/pcv003
Close - Share Icon Share
Abstract
The effects of Ca(NO3)2, KF and okadaic acid (OA) on cell hydraulic responses to NaCl were examined in roots of Arabidopsis thaliana wild-type plants and compared with plants overexpressing plasma membrane intrinsic protein PIP2;5. Root treatment with 10 mM NaCl rapidly and sharply reduced cell hydraulic conductivity (Lp) in the wild-type Arabidopsis plants, but had no effect on Lp in Arabidopsis plants overexpressing PIP2;5, suggesting that changes in protein and aquaporin gene expression were among the initial targets responsible for the inhibition of Lp by NaCl. The down-regulation of PIP transcripts after 1 h exposure to 10 mM NaCl was likely a significant factor in the reduction of Lp. The effect of NaCl on Lp in the wild-type plants was abolished when the NaCl-treated roots were subsequently exposed to 5 mM KF, 5 mM Ca(NO3)2 and 5 µM OA. The reduction of Lp by 5 mM KF could not be prevented by treatment with 5 mM Ca(NO3)2 in both wild-type and PIP2;5-overexpressing plants. However, 5 µM OA, which was added following NaCl or KF treatment, completely reversed Lp within several minutes. The results provide evidence for high sensitivity of aquaporin-mediated water transport to relatively low NaCl concentrations and point to the phosphorylation and/or dephosphorylation processes as those that are likely responsible for the protection of Lp by fluoride and calcium treatments against the effects of NaCl.
Introduction
Inhibition of root hydraulic conductivity is among the most sensitive early responses of plants to NaCl (Boursiac et al. 2005, Lee et al. 2010, Sutka et al. 2011). This effect has been attributed to the inhibition of cell hydraulic conductivity (Lp) through altered expression and (or) activity of aquaporins (Martínez-Ballesta et al. 2003, Alleva et al. 2006, Sutka et al. 2011). It has been long known that calcium (Ca) can ameliorate NaCl effects in many plants by preventing the accumulation of intracellular sodium (Nakamura et al. 1992), resulting in improved growth and water balance (Ehret et al. 1990, Azaizeh et al. 1992, Reid and Smith 2000). It has also been reported that some of the effects of NaCl on plants can be alleviated by fluoride (Calvo-Polanco et al. 2009, Lee et al. 2010). Although Ca and fluoride may affect water relations in plants exposed to NaCl through a number of different processes including root ion fluxes (Rubio et al. 2003, Shabala et al. 2003, Shabala et al. 2006), regulation of aquaporin-mediated water transport may play a role in these responses (Carvajal et al. 2000, Martínez-Ballesta et al. 2008, Lee et al. 2010, Horie et al. 2011, Katsuhara et al. 2011).
The role of Ca in aquaporin-mediated water transport of plants may involve complex processes. Increased cytosolic concentrations of Ca, which are usually associated with stress perception, may lead to aquaporin closure (Alleva et al. 2006, Martínez-Ballesta et al. 2008). It has also been reported that Ca stabilized the closed pore conformation and inhibited the plasma membrane intrinsic protein (PIP) activity (Maurel 2007, Verdoucq et al. 2008). However, when Ca concentrations were reduced by NaCl treatment, additional calcium applications resulted in up-regulation of aquaporin activity in Capsicum annuum and Arabidopsis (Martínez-Ballesta et al. 2008, Lee et al. 2012). Ca deficiency in plants subjected to NaCl stress could also affect aquaporin function through Ca-dependent phosphorylation and (or) dephosphorylation processes (Weaver et al. 1991, Johansson et al. 1996, Johansson et al. 1998, Vera-Estrella et al. 2004, Martínez-Ballesta et al. 2008). The function of aquaporins could also be affected by the loss of membrane integrity caused by the displacement of Ca from the cell membranes in NaCl-affected plants (Rengel 1992, Murata et al. 2000).
Ca is a crucial intracellular messenger and its homeostasis can change rapidly as a result of hormonal and environmental stimuli (Gilroy et al. 1993, Marschner 1995, White and Broadley 2003). Precipitation or binding of Ca in cells by chemical agents such as fluoride is among the primary factors responsible for their toxicity (Zwiazek and Shay 1987, Fornasiero 2001, Mackowiak et al. 2003). Fluoride is a potent inhibitor of many enzymes including phosphatases (Zwiazek and Shay 1988b), and regulates protein phosphorylation in cell membranes (Chang and Kuafman 2000, Struglics et al. 2000). Inhibition of aquaporin-mediated water transport was proposed to be the main process responsible for the inhibition of whole root and root cell hydraulic conductivities by fluoride (Kamaluddin and Zwiazek 2003, Lee et al. 2010). Interestingly, fluoride-induced injuries in plants can be partly prevented by NaCl (Calvo-Polanco et al. 2009, Calvo-Polanco et al. 2011) and, in roots exposed to NaCl, the inhibition of Lp could be completely reversed by 2 mM NaF and 2 mM KF treatments (Lee et al. 2010).
In the present study, we examined the effects of Ca, fluoride and okadaic acid (OA) on NaCl-induced inhibition of cell water transport in roots of the wild-type Arabidopsis thaliana and in Arabidopsis plants overexpressing PIP2;5 to better understand the role of aquaporins in these responses. OA is a commonly used protein phosphatase inhibitor (Cohen and Cohen 1989) and was shown to reverse the low temperature-induced inhibition of Lp in Arabidopsis (Lee et al. 2012). In the present study, we focused on PIP2;5 since this aquaporin was responsible for maintaining high water permeability in Arabidopsis root cells subjected to low temperature stress (Jang et al. 2007, Lee et al. 2012). We examined the responses of plants subjected to relatively low concentrations of NaCl (10 mM) to minimize the osmotic effects. We hypothesized that (i) aquaporin-mediated water transport is among the early processes affected by NaCl and, therefore, the effects of NaCl on cell water transport could be alleviated by the overexpression PIP2;5; and (ii) Ca and fluoride affect cortical cell water transport in roots exposed to NaCl through phosphorylation events.
Results
Cell hydraulic conductivity (Lp)
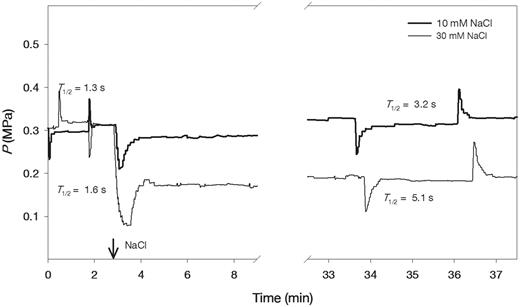
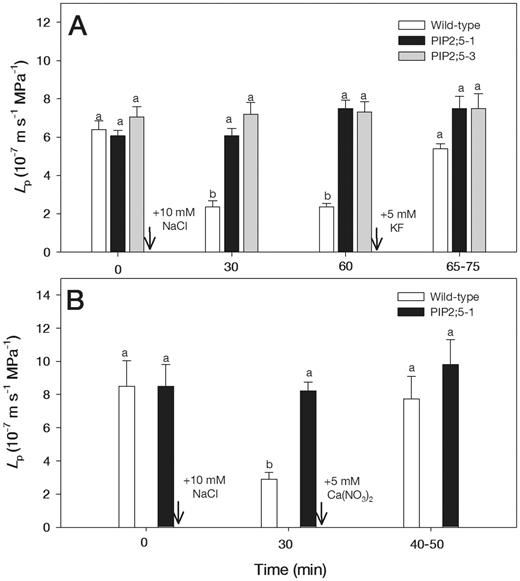
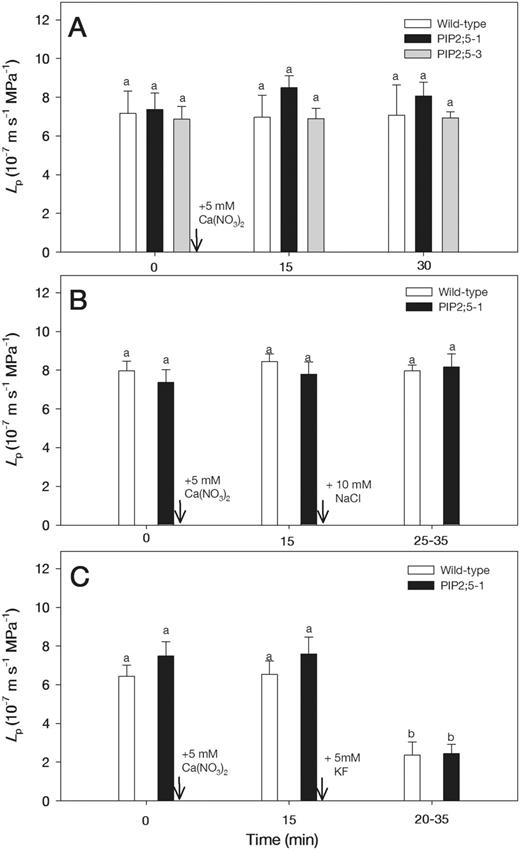
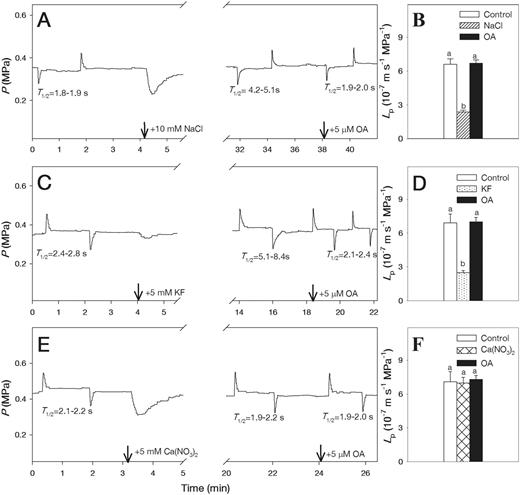
Typical hydrostatic pressure relaxation and biphasic osmotic response curves are shown for two cells from the roots which were subjected to 10 and 30 mM NaCl (Fig. 1). There was no significant change in turgor pressure (P) of cells treated with 10 mM NaCl; however, in cells treated with 30 mM NaCl, turgor pressure decreased from approximately 0.3 MPa to 0.18 MPa and remained constant over time (Fig. 1). The half-time of water exchange (T1/2) rapidly increased from 1.3 to 3.2 s after adding 10 mM NaCl and from 1.6 to 5.1 s after adding 30 mM NaCl (Fig. 1). The T1/2 values after adding 10 and 30 mM NaCl increased from 1.5 ± 0.1 to 3.7 ± 0.6 s and from 1.4 ± 0.1 to 4.3 ± 0.5 s, respectively (means ± SE, n = 6). The treatments with 10 mM NaCl decreased Lp by about 3-fold in the wild-type plants, but did not alter Lp in PIP2;5-overexpressing plants during the measurement times of 30–60 min (Fig. 2A). Therefore, NaCl was applied for 30 min to examine the combined effects of Ca(NO3)2 and 5 mM KF. When 5 mM KF was added following the 10 mM NaCl treatment, Lp values in the wild-type plants returned to close to the levels that were measured prior to NaCl treatment (Fig. 2A). Similarly, 5 mM Ca(NO3)2 reversed the inhibition of Lp caused by the 10 mM NaCl treatment in the wild-type plants (Fig. 2B). However, 5 mM KF and 5 mM Ca(NO3)2 did not affect Lp in NaCl-treated roots of PIP2;5-overexpressing plants (Fig. 2A, B).
Typical examples of hydrostatic and osmotic pressure relaxations in cortical cells of wild-type Arabidopsis roots in the presence and absence of 10 and 30 mM NaCl. NaCl was provided at the time indicated by the arrow. The half-time of water exchange (T1/2) values in root cortical cells on the left are before and on the right after NaCl treatment.
Effects of 5 mM KF (A) and 5 mM Ca(NO3)2 (B) on Lp in wild-type and PIP2;5-overexpressing Arabidopsis plants which were first exposed to 10 mM NaCl (A, B). The measurements were carried out on the same cells before and after adding treatment solutions. Arrows show the times when treatments were initiated. Means ± SE (n = 6–8) are shown. Different letters indicate statistically significant differences between treatments (P ≤ 0.05). The data were analyzed by ANOVA followed by Tukey’s multiple comparison.
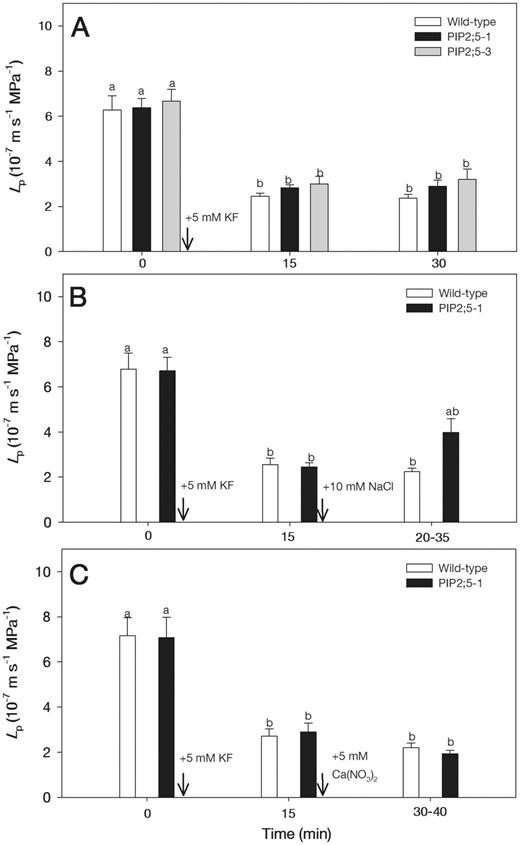
When the roots were subjected to 5 mM KF for 15 and 30 min, Lp values were reduced by about 3-fold both in the wild-type and PIP2;5-overexpressing plants (Fig. 3A). Therefore, following addition of KF for 15 min, NaCl and Ca(NO3)2 were applied to examine their combined effects on Lp. The decrease in Lp of KF-pre-treated roots was not affected in the wild-type plants by adding 10 mM NaCl (Fig. 3B). However, in the PIP2;5-overexpressing plants, there was a slight recovery of Lp following the 10 mM NaCl treatment (Fig. 3B). The decrease of Lp in roots treated with 5 mM KF was not further aggravated in both of wild-type and PIP2;5-overexpressing plants by the addition of 5 mM Ca(NO3)2 (Fig. 3C).
Effects of 5 mM KF (A), 5 mM KF followed by 10 mM NaCl (B) and 5 mM KF followed by 5 mM Ca(NO3)2 (C) on Lp in wild-type and PIP2;5-overexpressing Arabidopsis plants. Arrows show the times when treatments were initiated. Means ± SE (n = 6) are shown. Different letters indicate statistically significant differences between treatments (P ≤ 0.05). The data were analyzed by ANOVA followed by Tukey’s multiple comparison.
The treatment of roots with 5 mM Ca(NO3)2 for 15 and 30 min did not significantly affect Lp in the wild-type and PIP2;5-overexpressing plants (Fig. 4A). Since Lp values in the 5 mM Ca(NO)3 treatment remained constant for 30 min, Ca(NO3)2 was added for 15 min to examine the combined effects of NaCl and KF. When the Ca(NO3)2-treated roots were then subjected to 10 mM NaCl, Lp remained unaltered in the wild-type and PIP2;5-overexpressing plants (Fig. 4B). In contrast, the pre-treatment of roots with 5 mM Ca(NO3)2 did not protect Lp against the decline in the wild-type and PIP2;5-overexpressing plants when they were subsequently treated with 5 mM KF (Fig. 4C).
Effects of 5 mM Ca(NO3)2 (A), 5 mM Ca(NO3)2 followed by 10 mM NaCl (B) and 5 mM Ca(NO3)2 followed by 5 mM KF (C) on Lp in wild-type and PIP2;5-overexpressing Arabidopsis plants. Arrows show the times when treatments were initiated. Means ± SE (n = 6) are shown. Different letters indicate statistically significant differences between treatments (P ≤ 0.05). The data were analyzed by ANOVA followed by Tukey’s multiple comparison test.
The increases in T1/2 and a corresponding decreases in Lp following 10 mM NaCl and 5 mM KF treatments were reversed to the pre-exposure values by the addition of 5 µM OA (Fig. 5A–D). When the roots were subjected to OA alone, the values of the elastic modulus (ε) and T1/2 were not affected in the treated cortical cells (Supplementary Fig. S1). The application of OA to the roots that had been first subjected to the 5 mM Ca (NO3)2 treatments did not affect the T1/2 and Lp values (Fig. 5E, F).
Pressure probe traces showing effects of NaCl (A), KF (C) and Ca(NO3)2 (E) followed by 5 µM okadaic acid (OA) on half-time of water exchange (T1/2) values (left column) and cell hydraulic conductivity (Lp) values (B, D, F) (right column) in wild-type Arabidopsis roots. Means ± SE (n = 6) are shown. Different letters indicate statistically significant differences between treatments (P ≤ 0.05). The data were analyzed by ANOVA followed by Tukey’s multiple comparison test.
To account for possible effects of the counterions, we tested the effects of 10 mM KCl, 10 mM LiCl and 5 mM KNO3 in the wild-type plants, and found no effects of these solutions on Lp (data not shown).
Expression profiles of PIP genes
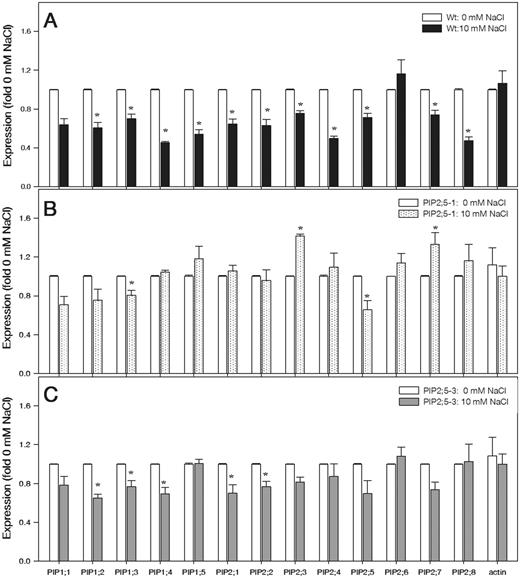
With the exception of PIP1;1 and PIP2;6, the PIP transcript abundance was significantly reduced in wild-type roots subjected to 10 mM NaCl treatment for 1 h compared with 0 mM NaCl (control) (Fig. 6A). In the PIP2;5 overexpression plants, the effect of 10 mM NaCl on PIP transcript abundance was relatively small (Fig. 6B, C). The transcript abundance of the reference gene (actin) was not significantly affected by the 10 mM NaCl treatment (Fig. 6A–C). The PIP transcript levels of transgenic Arabidopsis plants constitutively overexpressing PIP2;5 under the control of the Cauliflower mosaic virus 35S promoter (35S::PIP2;5 plants) (Jang et al. 2007) were confirmed by reverse transcription–PCR (RT–PCR) analysis (Supplementary Fig. S2). The amplified bands were present in the PIP2;5 overexpression lines and were not detected in wild-type plants (Supplementary Fig. S2).
Transcript levels of 13 PIP genes in the roots of wild-type Arabidopsis (A) and in the overexpression PIP2;5-1 (B) and PIP2;5-3 (C) lines of plants exposed to 0 (control) and 10 mM NaCl for 1 h. The transcript levels of 13 PIP genes were plotted as the relative expression (fold) of the control (0 mM NaCl). The transcript level of actin used as a reference is shown. Means ± SE (n = 3) from three independent experiments are shown. The data were analyzed by unpaired t-test, and asterisks above the bars indicate statistically significant differences (P ≤ 0.05).
Discussion
The treatment of roots with 10 mM NaCl rapidly inhibited Lp in wild-type Arabidopsis, but not in plants overexpressing PIP 2;5, indicating that the water transport mediated by PIP2;5 was among the initial targets of NaCl. It is interesting that the overexpression of PIP2;5 also protected the root PIP transcripts from the decrease observed in the wild-type plants treated with 10 mM NaCl, suggesting that the effect of NaCl on PIP gene expression may be a secondary response to water flow inhibition. Rapid regulation of various aquaporin genes in response to salt stress has been reported for several plant species (Suga et al. 2002, Aharon et al. 2003, Katsuhara et al. 2003, Boursiac et al. 2005, Aroca et al. 2009, Muries et al. 2011). However, the consequences of these changes to water relations of salt-stressed plants are not clear. In tobacco, salt stress resistance was not affected by the overexpression of PIP1b (Aharon et al. 2003) and AtPIP2;5 (Jang et al. 2007). In rice overexpressing HvPIP2;1, growth was shown to be more sensitive to salt stress compared with the wild-type plants (Katsuhara et al. 2003). Similarly, in most of the studies, overexpression of different aquaporins made the plants more susceptible to drought (Aharon et al. 2003, Jang et al. 2007). Since NaCl-induced stress is complex and involves both direct ion toxicity and osmotic effects (Martínez-Ballesta et al. 2008, Silva et al. 2008, Wan 2010), it should be expected that the function of aquaporins would also be affected by these two factors. Since the initial ionic effects of NaCl on water transport are poorly understood, we focused in the present study on the effects of relatively low 10 mM NaCl concentration to examine the responses of cell water transport before significant osmotic events occur. We demonstrated that 10 mM NaCl rapidly and strongly decreased Lp in the wild-type plants and that this decrease could be prevented and reversed by exposing the roots to 5 mM Ca(NO3)2. This corroborates the results of the protoplast swelling assays carried out in pepper (Capsicum annum), in which osmotic water permeability was made less sensitive to 50 mM NaCl by exposing the plants for 3 d to 10 mM CaCl2 (Martínez-Ballesta et al. 2008). These results suggest that the threshold cytosolic Ca concentrations, which were required to maintain the function of aquaporins responsible for transcellular water transport, were likely reduced by NaCl. Maintaining critical Ca levels may be required to keep the water channels open through gating processes such as phosphorylation (Azad et al. 2004, Martínez-Ballesta et al. 2008), reinforcing the view that aquaporin phosphorylation may be among the initial targets of NaCl stress.
Fluoride lowers the cytosolic Ca concentration and acts as a strong inhibitor of protein dephosphorylation (Gerbeau et al. 2002, Mackowiak et al. 2003). In earlier studies, either increases (Gerbeau et al. 2002) or decreases (Kamaluddin and Zwiazek 2003, Calvo-Polanco et al. 2009, Lee et al. 2010) of root and cell hydraulic conductivity by fluoride treatment were reported. Although the exact process responsible for the reported effects on hydraulic conductivity are not clear, it is interesting that the addition of fluoride to the roots completely reversed the inhibition of Lp in NaCl-treated Pinus banksiana roots (Lee et al. 2010). Ca affects water transport in a complex manner and may inhibit the PIP activity by stabilizing the closed conformation of the water channel pore (Maurel 2007, Verdoucq et al. 2008) or it may increase the water channel activity through Ca-dependent aquaporin phosphorylation (Martínez-Ballesta et al. 2008, Lee et al. 2012). Therefore, we examined the effects of Ca on Lp in combination with NaCl and KF to shed more light on the regulation of PIP function. Since Ca reacts with fluoride to produce insoluble CaF2, we expected that 5 mM KF treatment would negate the ameliorating effects of 5 mM Ca(NO3)2 in NaCl-treated roots. Similarly to earlier reports (Kamaluddin and Zwiazek 2003, Lee et al. 2010), 5 mM KF strongly reduced Lp in Arabidopsis roots, but, unlike NaCl, fluoride also affected Lp in plants overexpressing PIP2;5 and these reductions could not be reversed by the 5 mM Ca(NO3)2 treatment. Therefore, the inhibition of Lp by fluoride is likely more potent compared with NaCl. Since fluoride action involves Ca precipitation, we cannot exclude the possibility that precipitation of Ca outside of the root surface could further aggravate the Lp reduction (Mackowiak et al. 2003). However, it is intriguing that the inhibition of Lp by 10 mM NaCl in the wild-type plants was almost completely reversed by the subsequent 5 mM KF and 5 mM Ca(NO3)2 treatment. It is also interesting that in the PIP2;5-overexpressing plants, Lp was not affected by 5 mM KF when the roots had been previously treated with 10 mM NaCl. Therefore, in addition to aquaporin expression, regulation of aquaporin-mediated water transport appears to involve complex gating mechanisms. Cell hydraulic conductivity could also be potentially affected by changes in cell rigidity. However, in the present study, cell elastic modulus was not affected by the Ca(NO3)2 or KF treatments (Supplementary Fig. S1; Supplementary Table S2) and, therefore, cell wall elasticity was unlikely to be a significant factor affecting cell water transport properties in treated roots. The effects of fluoride, NaCl and Ca on cell water transport may be indirect and related to the effects on membrane integrity, changes in membrane lipid composition, internalization and protein stability (Zwiazek and Shay 1987, Zwiazek and Shay 1988a, Allakhverdiev et al. 2000, Kaya et al. 2001, Boursiac et al. 2005, Boursiac et al. 2008). These aspects will require more attention in future studies.
The reductions of Lp in the NaCl and KF treatments were reversed to the pre-treatment level after exposing the roots to 5 µM OA, a protein phosphatase inhibitor (Cohen and Cohen 1989, Johansson et al. 1998, Horie et al. 2011). However, OA did not affect Lp in roots previously treated with Ca(NO3)2. These results support the conclusions of the studies involving PIP2;5 overexpression plants that both Ca-dependent phosphorylation events and regulation of aquaporin gene expression are likely to be involved in the responses of aquaporin-mediated water transport to NaCl and KF (Weaver et al. 1991, Azad et al. 2004, Lee et al. 2012). While our evidence clearly demonstrates that KF, Ca(NO3)2 and OA alter plant responses to low concentrations of NaCl, finding the precise mechanisms behind these responses is still challenging.
In our study, 1 h treatment with 10 mM NaCl reduced root transcript levels of all PIPs in the wild-type plants, with the exception of PIP1;1 and PIP2;6. In several studies, NaCl down-regulated multiple genes of aquaporins (Martínez-Ballesta et al. 2003, Boursiac et al. 2005), suggesting a link to the reductions in root water transport. On the other hand, NaCl was also reported to increase root expression of aquaporins in Arabidopsis (Jang et al. 2004). The discrepancy between these findings may be due to differences in plant age, growth conditions, intensity of stress and timing differences. In the earlier report (Boursiac et al. 2005), the PIP transcript levels were highly stable in plants treated for 2 h with NaCl, and an inhibition of PIP gene expression occurred only after the reduction of root hydraulic conductivity (>2 h). However, the overall abundance of AtPIP1 protein decreased as early as 30 min following exposure to NaCl. This indicates that NaCl induced protein instability and that changes in protein expression can react rapidly in response to stress and affect root water transport (Voicu and Zwiazek 2004, Boursiac et al. 2005). In addition, NaCl-induced changes in trafficking of PIP1 and PIP2 isoforms between the plasma membrane and intracellular compartments reduced the abundance of PIPs at the plasma membrane and led to the reduction in root hydraulic conductivity (Boursiac et al. 2008, Prak et al. 2008, Li et al. 2011, Sorieul et al. 2011, Luu et al. 2012).
The results of the present study suggest that inhibition of Lp in plants exposed to 10 mM Nacl may be due to the effect on aquaporin function and protein phosphorylation and/or dephosphorylation processes. Most of the aquaporins are small molecular mass proteins (25–35 kDa) and the activity of many aquaporins is affected by phosphorylation and dephosphorylation events (Maurel et al. 1995, Kjellbom et al. 1999, Johansson et al. 2000, Santoni et al. 2006, Prak et al. 2008, Horie et al. 2011). Phosphorylation of plant aquaporins at the serine residue is catalyzed by the Ca-dependent protein kinase (Johansson et al. 2000, Karlsson et al. 2003, Azad et al. 2004, Horie et al. 2011), and PIP2 phosphorylation in the loop B and C-terminal tail controls pore opening and closing (Johansson et al. 1996, Johansson et al. 1998, Prak et al. 2008). Aquaporin phosphorylation has also been found to facilitate water transport from the stem to the petals, while the dephosphorylation triggered the closure of water channels in plants subjected to the low temperature and salt treatments (Azad et al. 2004, Horie et al. 2011).
In conclusion, 10 mM NaCl rapidly and sharply reduced Lp in roots of the wild-type Arabidopsis plants, but had no effect on Lp in Arabidopsis plants overexpressing PIP2;5. The down-regulation of PIP transcript profiles and aquaporin phosphorylation may contribute to the reduction of Lp. The effect of NaCl on Lp was reversed by exposing the roots to 5 mM Ca(NO3)2, 5 mM KF and 5 µM OA. The OA treatment also reversed the effects of 5 mM KF on Lp, but had no effect on Lp in roots of plants treated with 5 mM Ca(NO3)2. The results demonstrate that (i) both aquaporin phosphorylation/dephosphorylation events and aquaporin expression patterns may be among the initial targets of low NaCl concentrations; and (ii) Ca and fluoride effects on cell water transport in NaCl-treated plants probably involve phosphorylation and dephosphorylation processes.
Materials and Methods
Plant material and growth conditions
Seeds of the wild-type (ecotype Columbia 0) A. thaliana and plants constitutively overexpressing Arabidopsis PIP2;5 (Jang et al. 2007) were sown in Jiffy pots (Jiffy Company Ltd.) for 2 d at 4°C and then transplanted to an aerated nutrient solution. The plants were grown for 21–25 d in 5 liter containers which were placed in the controlled-environment growth chamber under a 16 h photoperiod, approximately 350 µmol m−2 s−1 photosynthetic photon flux density (PPFD) and 23/21°C (day/night) temperature (Lee et al. 2012). The nutrient solution contained 1.25 mM KNO3, 1.5 mM Ca(NO3)2, 0.75 mM MgSO4, 0.5 mM KH2PO4, 50 µM H3BO3, 10 µM MnCl2, 2 µM ZnSO4, 1.5 µM CuSO4, 0.075 µM NH4Mo7O24 and 74 µM Fe-EDTA.
Cell pressure probe measurements and application of chemicals
When cortical cells of Arabidopsis roots had attained a steady half-time of water exchange across the cell membrane (T1/2) during the hydrostatic water flow, treatment solutions containing 10 mM NaCl, 5 mM KF, 5 mM Ca(NO3)2 and 5 µM OA were added to the circulating medium to examine their effects on the water relations parameters. Hydraulic parameters were continuously measured in the absence of NaCl and compared with those measured after adding NaCl, followed by Ca(NO3)2 , KF or OA on the same individual cells. These measurements were repeated when the roots were subjected first to KF followed by NaCl, Ca(NO3)2 or OA and when the roots were subjected first to Ca(NO3)2 followed by NaCl, KF or OA.
RNA extraction, RT–PCR and real-time RT–PCR
Total RNA was extracted from the frozen root samples using the Plant RNeasy extraction kit (Qiagen). The concentration of RNA was quantified spectrophotometrically using a NanoDrop 1000 apparatus, and 5 µg of total RNA was separated on a 1.2% formaldehyde agarose gel to check the concentrations and to monitor the integrity of samples. A 1 µg aliquot of total RNA was used in an RT–PCR system (Qiagen) together with gene-specific primers; PIP2;5 (Forward, 5′-GGGATCCCAATGACGAAGGAAGTGG-3′; Reverse, 5′-CTCGAGTTAAACGTGAGGCTGGCTC-3′) and actin (Forward, 5′-CAGCAGAGCGGGAAATTGTAAGAG-3′; Reverse, 5′-TTCCTTTCAGGTGGTGCAACGAC-3′). Real-time RT–PCR was performed using a Biosystems 7500 real-time thermal cycling system (Applied Biosystems) with the QuantiTect SYBR Green RT-PCR kit (Qiagen). We used 3′-untranslated region gene-specific primers (Jang et al. 2004) except for PIP1;3, PIP1;5, PIP2;1, PIP2;3 and PIP2;7 (Supplementary Table S1), and quantified the abundance of PIP transcripts using real-time PCR analysis. After normalization of the RNA content using the actin gene expression pattern in each sample, the expression levels of each of the 13 PIP genes in wild-type and PIP2;5-overexpressing Arabidopsis plants which were grown at 0 and 10 mM NaCl for 1 h in solution culture were checked. These experiments were repeated at least three times, and the histograms represent the mean values and standard error bars of different experiments conducted with different RNA preparations.
Statistical analyses
The data were analyzed using analysis of variance (ANOVA) to compare the effects on the cell hydraulic conductivity, and the unpaired t-test was used for aquaporin gene expression. Statistical analyses were performed using SigmaPlot V 11.0 (Systat Software Inc.) at the α = 0.05 level.
Funding
This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant to J.J.Z.
Acknowledgments
We thank Professor Hunseung Kang at the Chonnam National University, Gwangju, Korea for the seeds of Arabidopsis thaliana overexpressing PIP2;5, and Dr. J.Y. Jang for help with RT–PCR analysis.
Disclosures
The authors have no conflicts of interest to declare.
Abbreviations
- A
cell surface area
- ε
cell elastic modulus
- Lp
cell hydraulic conductivity
- OA
okadaic acid
- PIP
plasma membrane intrinsic protein
- P
turgor pressure
- π
osmotic pressure of the cell
- RT–PCR
reverse transcription–PCR
- T1/2
half-time of water exchange
- V
cell volume