-
PDF
- Split View
-
Views
-
Cite
Cite
Hiroki Konno, Takeshi Nakane, Masasuke Yoshida, Hanayo Ueoka-Nakanishi, Satoshi Hara, Toru Hisabori, Thiol Modulation of the Chloroplast ATP Synthase is Dependent on the Energization of Thylakoid Membranes, Plant and Cell Physiology, Volume 53, Issue 4, April 2012, Pages 626–634, https://doi.org/10.1093/pcp/pcs018
Close - Share Icon Share
Abstract
Thiol modulation of the chloroplast ATP synthase γ subunit has been recognized as an important regulatory system for the activation of ATP hydrolysis activity, although the physiological significance of this regulation system remains poorly characterized. Since the membrane potential required by this enzyme to initiate ATP synthesis for the reduced enzyme is lower than that needed for the oxidized form, reduction of this enzyme was interpreted as effective regulation for efficient photophosphorylation. However, no concrete evidence has been obtained to date relating to the timing and mode of chloroplast ATP synthase reduction and oxidation in green plants. In this study, thorough analysis of the redox state of regulatory cysteines of the chloroplast ATP synthase γ subunit in intact chloroplasts and leaves shows that thiol modulation of this enzyme is pivotal in prohibiting futile ATP hydrolysis activity in the dark. However, the physiological importance of efficient ATP synthesis driven by the reduced enzyme in the light could not be demonstrated. In addition, we investigated the significance of the electrochemical proton gradient in reducing the γ subunit by the reduced form of thioredoxin in chloroplasts, providing strong insights into the molecular mechanisms underlying the formation and reduction of the disulfide bond on the γ subunit in vivo.
Introduction
Chloroplast ATP synthase (CFoCF1) is known as a thiol enzyme; a disulfide bond located on the γ subunit is reduced by reducing equivalents supplied by the photosynthetic electron transfer reaction. The key protein mediating the transfer of the reducing equivalents from photosynthetic reactions to the disulfide bond on the target enzyme is thioredoxin (Trx) in the chloroplast stroma, identified in Escherichia coli in 1964 as a ribonucleotide reductase cofactor (Laurent et al. 1964). The significance of Trx in chloroplasts as a reducing equivalent mediator was first characterized in 1977 (Holmgren et al. 1977), and the basic concept of thiol modulation of chloroplast enzymes by the reduced form Trx was established by Buchanan et al. (Wolosiuk and Buchanan 1977). The catalytic moiety of the chloroplast ATP synthase, CF1, is a target of thiol modulation, and the role of Trx in activation of the ATP hydrolysis activity of the isolated CF1 was demonstrated in the presence of dithiothreitol (DTT: McKinney et al. 1979). The γ subunit of spinach CF1, possessing four cysteines in its amino acid sequence, is critical for this thiol modulation. Two of these cysteines (Cys199 and Cys205) are located in the central region of the subunit and have been shown to be important for redox regulation (Nalin and McCarty 1984, Miki et al. 1988). Interestingly, the region containing the two regulatory cysteines, the so-called ‘inserted sequence’, is comprised of a total of about 40 amino acid residues, and is only present in the γ subunit of chloroplast ATP synthases. Comparison of whole sequences of the γ subunit from various organisms shows that the γ subunit of cyanobacteria includes the insertion sequence consisting of 30 amino acids, though the region lacks the two regulatory cysteines (Werner-Grune et al. 1994, Hisabori et al. 2003), suggesting that is not redox regulated.
In the past two decades research has been primarily focused on the chemical reduction of the disulfide bond located on the γ subunit of the isolated CF1, and the relationship between reduction of the disulfide bond and the possible redox regulation of the activity has been investigated in detail (Dann and McCarty 1992, Soteropoulos et al. 1992, Hightower and McCarty 1996, Bald et al. 2000). The redox regulation of rotation of the γ subunit in the α3β3 hexamer was then visualized using the chimeric α3β3γ complex obtained from thermophilic bacteria, whose γ subunit was designed to contain the regulatory region of the spinach γ subunit in the central domain (Bald et al. 2001, Ueoka-Nakanishi et al. 2004), and, more recently, using the α3β3γ complex of thermophilic cyanobacteria containing the regulatory segment from the spinach γ subunit (Kim et al. 2011). However, there have been no in vivo reports to date pertaining to the reduction of the disulfide bond on the γ subunit of the CFoCF1 complex by reducing equivalents generated in vivo, although photosynthetic activation of CFoCF1 was indirectly measured using the electrochromic shift of carotenoid in chloroplasts (Kramer and Crofts 1989, Kramer et al. 1990). In addition, most studies concerning the activation of the thiol enzymes by reduction have historically been carried out in vitro, predominantly by way of the classic reducing agent DTT in the millimolar range or, more recently, the substitute Tris(2-carboxyethyl)phosphine.
In this study, particular focus was placed on reduction of the γ subunit by the ‘in vivo’ photosynthetic electron transport system, and reduction and reoxidation of the disulfide bond on the γ subunit in intact chloroplasts and in leaves was investigated. The combined methodologies led to the elucidation of the physiological relevance of this key enzyme: reduction of the γ subunit of CFoCF1 for enzyme activity, and oxidation for the maintenance of photosynthesis as a regulatory mechanism. In addition, the conditions required for the effective reduction and oxidation of the γ subunit in vivo were studied.
Results
Photoreduction of the chloroplast ATP synthase
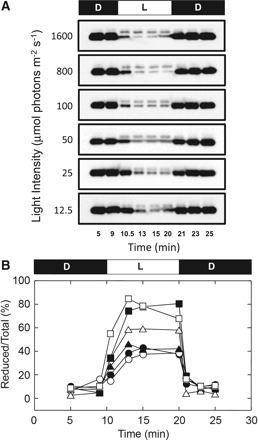
We first sought to determine whether completely reduced or oxidized CF1 γ subunit using standard chemical reduction or oxidation treatments could be separated and quantified by the 4-acetoamido-4′-maleimidylstilbene-2,2′-disulfonate (AMS) labeling method (Supplementary Fig. S1A). Following AMS labeling, the redox state of the γ subunit was measured by differences in the mobility of labeled protein bands on SDS–PAGE caused by modification of reduced cysteine residues with AMS. Since the signal intensities generated by Western blotting of the reduced and oxidized forms of the γ subunit were clearly different from each other due to the specificity of the polyclonal antibody applied to the experiments, the integrated band intensities using the same amounts of proteins were determined, respectively, and the averaged ratio of the band intensities 1 : 5.5 (reduced : oxidized) was determined (Supplementary Fig. S1A, Supplementary Table S1). This ratio value was used for calibration of the following studies. The photo-reduction level of the γ subunit was calculated as the ratio of the band intensities of the reduced form and that of the total γ subunits (reduced form plus oxidized form) shown in Fig. 1A. As a reference, we examined the redox change of fructose 1,6-bisphosphatase (FBPase), a well-known thiol enzyme found in the chloroplast stroma, and found that the signal intensities of the Western blots of the reduced and the oxidized form FBPase were equivalent (Supplementary Fig. S1B).
Dependency of photoreduction of CF1 γ in intact chloroplasts on light intensity. (A) Following incubation for 10 min in the dark, intact chloroplasts (0.2 mg Chl ml−1) were illuminated for 10 min at the light intensities indicated and then incubated for an additional 10 min in the dark. At appropriate time intervals, the sample was mixed directly with the thiol group labeling solution containing AMS, and the redox level of the γ subunit in the intact chloroplasts visualized using the CF1-γ antibody following SDS–PAGE. (B) The redox level of the γ subunit in intact chloroplasts was determined as described in the Materials and Methods from the band intensities shown in A. Filled circles, 12.5 μmol photons m−2 s−1; open circles, 25 μmol photons m−2 s−1; filled triangles, 50 μmol photons m−2 s−1; open triangles, 100 μmol photons m−2 s−1; filled squares, 800 μmol photons m−2 s−1; open squares, 1,600 μmol photons m−2 s−1.
By using the method described above, the difference in the level of photoreduction of the γ subunit of CF1 in intact chloroplasts under various light intensities was then studied (Fig. 1). Even at 12.5 or 25 μmol photons m−2 s−1, partial photoreduction of the γ subunit was observed. At a light intensity of >800 μmol photons m−2 s−1, the photoreduction level of the γ subunit was almost constant and saturated at around 80–90% of the total γ. The rate of reduction of the γ subunit after turning on the light was independent of the light intensity, and was slower than the rate of oxidation. It reached the maximal reduction level within 3 min, although rapid oxidation was observed immediately after turning off the light.
Effects of various inhibitors on reduction of the γ subunit.
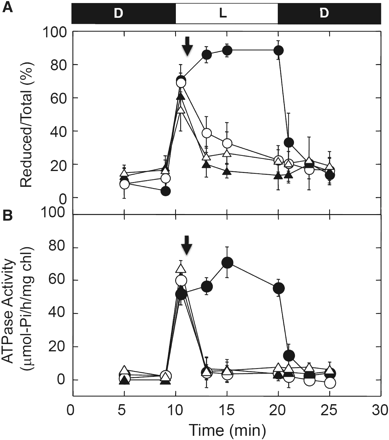
In order to characterize significant factors which determine the reduction level of the γ subunit, we investigated the effect of inhibitors of photosynthetic electron transport and an uncoupler. For this purpose, DCMU, a specific inhibitor which blocks the electron flow from PSII to plastoquinone, 2,5-dibromo-3-isopropyl-6-methyl-p-benzoquinone (DBMIB), a specific inhibitor that blocks the interaction between plastoquinone and cytochrome b6f, and the uncoupler, carbonylcyanide p-trifluoromethoxyphenylhydrazone (FCCP), were employed. After illumination of the chloroplasts with sufficient light intensity (800 μmol photons m−2 s−1) for 1 min, these inhibitors were added to the chloroplast preparation under light conditions. When the electrochemical gradient of proton (ΔμH+) was collapsed in thylakoids by addition of FCCP, the oxidation of γ was superior to reduction of γ. The oxidation of γ was, however, much slower than that observed following the transition from light to dark, and the full oxidation after addition of FCCP in the light required about 10 min (Fig. 2A). The effects of the electron transfer inhibitors, DCMU and DBMIB, were more pronounced and the γ subunit was immediately oxidized after their addition (Fig. 2A). Measurement of ATP hydrolysis activity (Fig. 2B) showed a similar tendency irrespective of the difference in the inhibitors, although the collapse of the ATP hydrolysis activity was faster than the oxidation of γ observed in Fig. 2A.
Effects of an uncoupler and electron transport inhibitors on photoreduction of the γ subunit. (A) Following incubation for 10 min in the dark, intact chloroplasts (0.2 mg Chl ml−1) were illuminated at 800 μmol photons m−2 s−1 for 10 min and then incubated for an additional 5 min in the dark (filled circles). At 1 min post-illumination, (filled triangles) 20 μM DBMIB, (open triangles) 10 μM DCMU and (open circles) 2.5 μM FCCP were added to the chloroplast solution in the light (downward-facing arrow), and the redox level of the γ subunit was determined at the time indicated. The results of three independent experiments were averaged. The size of the standard error is shown on each plot. (B) The effects of an uncoupler and electron transport inhibitors on ATP hydrolysis activity were examined. The intact chloroplast solution (70 μg Chl ml−1) was illuminated at 800 μmol photons m−2 s−1, and the inhibitors added to the chloroplasts as for A. The ATP hydrolysis activities were determined as described in the Materials and Methods. The results of three independent experiments were averaged and the size of the standard error is shown on each plot.
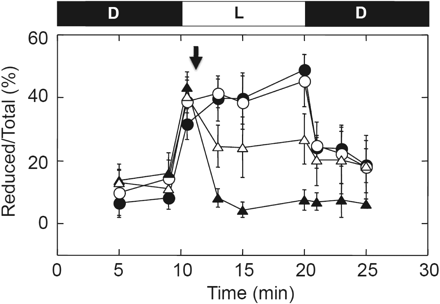
In contrast, the reduction level of FBPase, a typical chloroplast thiol enzyme, showed a different response to inhibitors; although rapid oxidation was induced by the addition of DBMIB, the uncoupler FCCP had no effect on the reduction level of FBPase (Fig. 3), indicating that transfer of reducing equivalents from the photosynthetic electron transport system to Trx was not inhibited by uncoupling. In contrast, the effect of DCMU was limited for the oxidation of FBPase, suggesting that linear electron flow is not a unique prerequisite for the reduction of Trx in chloroplasts.
Effects of uncoupler and electron transport inhibitors on photoreduction of FBPase. Following incubation for 10 min in the dark, intact chloroplasts (0.2 mg Chl ml−1) were illuminated at 800 μmol photons m−2 s−1 for 10 min and then incubated for an additional 5 min in the dark (filled circles). At 1 min post-illumination, (filled triangles) 20 μM DBMIB, (open triangles) 10 μM DCMU and (open circles) 2.5 μM FCCP were added to the chloroplast solution in the light (downward-facing arrow), and the redox level of FBPase was determined at the time indicated. The results of three independent experiments were averaged. The size of the standard error is shown on each plot.
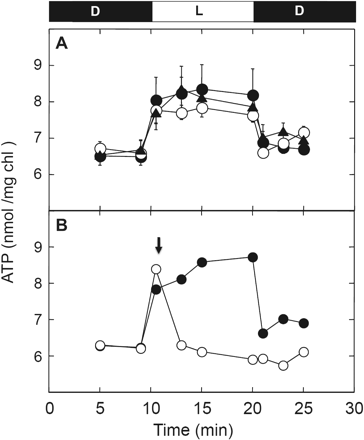
To investigate the relevance of the reduction of the γ subunit to ATP synthase activity in detail, ATP synthesis was measured under the following light intensities: 25, 100 and 800 μmol photons m−2 s−1 (Fig. 4), resulting in different reduction levels of the γ subunit (Fig. 1B). Interestingly, the chloroplast ATP concentration reached the same level under these different light intensities, indicating that ATP synthesis was promoted in chloroplasts irrespective of the redox level of the γ subunit. However, it is difficult to describe the change in ATP synthesis activity itself as the steady-state ATP level represents the balance between generation and hydrolysis of ATP. The ATP concentration in chloroplasts decreased to the baseline level immediately by addition of FCCP, due to the collapse of ΔμH+, which is required for ATP synthesis
ATP level in the intact chloroplasts under different light intensities. (A) Intact chloroplasts (0.2 mg Chl ml−1) were incubated for 10 min in the dark, illuminated at 25 μmol photons m−2 s−1 (filled triangles), 100 μmol photons m−2 s−1 (open circles) and 800 μmol photons m−2 s−1 (filled circles) for 10 min, and incubated for an additional 5 min in the dark. At the indicated time, a portion of the chloroplast samples was mixed directly with trichloroacetic acid, and the amounts of ATP determined as described in the Materials and Methods. The results of three independent experiments were averaged and the size of the standard error is shown on each plot. (B) Following incubation for 10 min in the dark, intact chloroplasts were illuminated at 800 μmol photons m−2 s−1 for 10 min and then incubated for an additional 5 min in the dark (filled circle). At 1 min post-illumination, 2.5 μM FCCP was added to the chloroplast solution in the light (downward-facing arrow, open circle).
Redox change in the γ subunit of CFoCF1 in field-grown spinach leaves
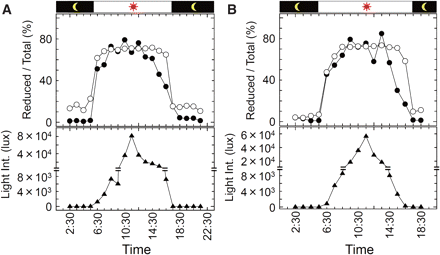
Finally, the daylight response of the redox state of the γ subunit of CFoCF1 in field-grown spinach leaves was investigated. Spinach plants grown in an outdoor planter for 8–9 weeks after germination were used for this experiment, and diurnal changes in redox levels of the thiol enzymes in leaves were monitored as described in the Materials and Methods (Fig. 5). Reduction of thiol enzymes initiated immediately after sunrise irrespective of the weather, and reached a maximal level of around 60–80% within 3 h. The light intensity required to reach the maximal level was about 100–150 μmol photons m−2 s−1 [0.6–1.0 × 104 lux by the luxmeter (since the sensor of the light meter is very sensitive to the direction of the light source and not applicable to the measurement in our experiments under the outside day–light conditions, light intensity was measured by the luxmeter in the following experiments)] (Fig. 5). Even though the light intensity outside further increased during daytime, the reduction levels of the γ subunit and FBPase were at near plateau levels and did not change significantly. Oxidation of these enzymes was observed in proportion to the decrease in light intensity, and the enzymes were converted into the fully oxidized form by sunset. In this process, oxidation of FBPase started apparently earlier than that of the γ subunit.
Photoreduction of thiol enzymes in field-grown spinach leaves. Field-grown spinach leaves in a planter were collected at the time indicated and the redox states of the thiol enzymes were visualized by the described method. The redox level of the γ subunit (open circles), FBPase (filled circles) and change of the light intensity (filled triangles) are indicated. The results of two independent measurements are shown; (A) the sampling was carried out on October 30, 2008. The weather was occasionally cloudy throughout the day. (B) The sampling was carried out on November 11, 2008. The weather was cloudy all day.
Discussion
The basic concept of photosynthetic activation of thiol enzymes in chloroplasts has been established through a number of seminal pieces of research (see the reviews by Schurmann 2003, Buchanan and Balmer 2005). Chloroplast ATP synthase has historically been a well-known thiol enzyme, whose ATP hydrolysis activity is regulated by the chloroplast Trx-f (Mills et al. 1980, Schwarz et al. 1997), and the relationship between the reduction of the disulfide bond on the γ subunit and activation of the enzyme activity has been studied in depth. In addition, Kramer et al. (1990) reported activation of the sunflower CFoCF1 in the light based on the measurement of electrochromic shifts in leaf thylakoids, concluding that the catalytic activation of CFoCF1 is not rate limiting to the induction of carbon assimilation under field conditions during a natural dark-to-light transition at sunrise. This study represents the first effort to determine the redox state of CFoCF1 in vivo, though the actual redox states of CF1 could not be determined in their measurements. To date, no direct evidence exists relating to the photosynthetic reduction of the γ subunit of CF1 in leaves of plants in the natural environment. To confirm this phenomenon, direct assessment of the reduction level of the γ subunit using the thiol modifier AMS and a specific antibody raised against the regulatory region of the γ subunit was employed in this study.
When the photosynthetic reduction of γ in intact spinach chloroplasts was measured, the maximum reduction level of the γ subunit was estimated to be 80–90% (Fig. 2A), although the thiol enzyme FBPase was found to be reduced by only 40–50% under the same conditions (Fig. 3). Although the cause of the fluctuation in reduction level observed using intact chloroplasts remains unclear, we observed that reduction of both the γ subunit and FBPase in plant leaves was 70–80% when planter-grown spinach leaves were directly frozen by liquid nitrogen immediately after the sampling under sunlight (Fig. 5).
The major function of γ subunit reduction is thought to be to lower the energetic threshold for the ATP synthesis reaction (Ketcham et al. 1984, Hangarter et al. 1987, Junesch and Graber 1987). However, our reduction experiments using intact chloroplasts clearly show that reduction of the γ subunit never occurs without ΔμH+ (Fig. 2A), as suggested by previous indirect measurements reported by Kramer et al. (1990). Since reduction did not require strong light intensity as reported, reduction of γ never acts as a rate-limiting step for ATP synthesis in this context (see Fig. 1). In addition, direct measurement of the ATP concentration in the intact chloroplasts suggested that ATP synthesis itself initiated even under insufficient light conditions for the full reduction of the γ subunit, and reached a significant level within a few minutes (Fig. 4). Note that the relevant connection between ATP synthesis activity and the reduction level of the γ subunit should be further studied since the net ATP level we measured is the sum of the synthesis and consumption of ATP in chloroplasts. Nevertheless, the result obtained here again suggests that the reduction of the γ subunit in chloroplasts is not a prerequisite for efficient ATP synthesis in the light. This conclusion corroborates previous reports that ATP synthase can become active and support high rates of ATP synthesis even in the oxidized state (Junesch and Graber 1987, Ponomarenko et al. 1999), though Wu et al. recently reported that the cfq mutant whose γ subunit was more difficult to reduce by Trx showed a lower efficiency of ATP formation (Wu et al. 2007, Wu and Ort 2008). This cfq mutant was originally obtained from a random mutagenesis study of Arabidopsis thaliana, when those mutant plants were selected that grew poorly under low irradiance but performed satisfactorily at high irradiance (Gabrys et al. 1994). Consequently the resulting cfq mutant showed a phenotype in which the γ subunit was hardly reduced. The reduction level of γ returned to the basal level when FCCP was added in the light (Fig. 2). The slow oxidation observed might be due to the partial reducing effect of the reduced form of Trx in chloroplasts, since the photosynthetic reduction of Trx occurs even in the presence of FCCP, as seen by photosynthetic reduction of FBPase (Fig. 3). Johnson and McCarty (2002) reported that the large conformational change of the C-terminal part of the ε subunit is induced by the formation of ΔμH+ in the thylakoid membranes. In addition, the reduction of the γ subunit is affected by the presence or the absence of the ε subunit in the case of the isolated CF1 (Richter et al. 1985, Konno et al. 2004). Taken together, the reduction of the γ subunit in the presence of ΔμH+ shown in our study (Fig. 2A) must be accomplished via the conformational change of the ε subunit. The ε subunit is known as an intrinsic inhibitor protein for ATP hydrolysis activity of CF1, and the C-terminal helices are significant for both the inhibition and the coupling (Imashimizu et al. 2011). In addition, the ε subunit acted as a strong inhibitor of ATP hydrolysis activity when the subunit was added to the enzyme complex without ε in vitro (Richter et al. 1984, Konno et al. 2006). Therefore, the change in activity of the membrane-bound complex must be much more complex than the reduction kinetics. This must be the reason why ATP hydrolysis activity did not follow the oxidation process observed by the addition of inhibitors (Fig. 2A, B). In contrast, the gradual oxidation of γ initiated by the addition of inhibitors (Fig. 2A) implies that disulfide bond formation on the γ subunit occurs spontaneously in chloroplasts irrespective of the light/dark conditions and the reduced form Trx conclusively shifts the equilibrium between reduced and oxidized γ to the former. As suggested previously by measurement of the change in ATP hydrolysis activity using thylakoid membranes (Mills and Mitchell 1982) and protoplasts (Quick and Mills 1986), the observed tendency of the equilibrium to shift to the oxidation state of CF1 in chloroplasts is likely to confer the advantage of the ability to shut down inadequate ATP hydrolysis activity, thus avoiding futile ATP consumption under conditions where photosynthesis does not occur.
Although the reduction level of thiol enzymes should be determined by the balance between the photosynthetic reduction and the spontaneous oxidation as mentioned, the factors involved in the oxidation process of thiol enzymes such as FBPase even at midday (Fig. 5), and the γ oxidation in the presence of FCCP (Fig. 2) remain unclear. From the study of the diurnal dynamics of photosynthesis in plants, midday depression of photosynthesis is a well-known phenomenon (Muraoka et al. 2000, Spunda et al. 2005, Kets et al. 2010), and it would be important to study the potential higher order regulatory process for the thiol enzyme oxidation. One of the strong candidates involved in this oxidation process was proposed to be Trx itself from the study of redox change of the chloroplast ATP synthase (Mills and Mitchell 1982). However, since slow oxidation of the γ subunit was observed in the presence of FCCP in the light, and FBPase was reduced even in the presence of FCCP (Fig. 3), most of the chloroplast-localized Trx must be in the reduced form under the experimental conditions used herein. Other oxidants and/or Trx can therefore be presumed to act as electron acceptors and be responsible for oxidation of the γ subunit, and thus the electron acceptor for the oxidation of thiol enzymes in chloroplasts in the dark should be the subject of further investigation.
ATP synthase is now known as a motor enzyme that shows rotary motion during catalysis, with the γ subunit constituting the central axis subunit in the complex rotating against the surrounding α and β hexagon. Reduction of the disulfide bond located on the γ subunit may therefore easily regulate rotation through a change in its conformation (Bald et al. 2001, Ueoka-Nakanishi et al. 2004). Interestingly the region in the γ subunit responsible for redox-dependent conformational change is likely to be located near subunit c, which forms the ring structure with 14 protomers (Seelert et al. 2000) in the thylakoid membranes. Since subunit c contains the critical glutamate residue required for proton translocation during catalysis, it is feasible that the conformational change of the γ subunit or susceptibility of Trx to this subunit would be indirectly affected by the protonation of the glutamate residues in the c-ring, or the conformational change of the exposed part of γ from the α3β3 hexagon by rotation (Vik and Antonio 1994, Engelbrecht and Junge 1997). As the proteolysis of γ was affected by proton translocation via the c-ring (Sugiyama and Hisabori 2003), a certain conformational change of γ must be induced by protonation at the c subunit.
Materials and Methods
Preparation of intact chloroplasts
Intact chloroplasts were isolated according to the method described by Motohashi et al. (2001): 100 g of market spinach leaves were homogenized for 10 s in a mixer with 250 ml of grinding solution containing 330 mM sorbitol, 5 mM sodium ascorbate, 0.05% (w/v) bovine serum albumin, 2 mM EDTA, 50 mM HEPES-KOH (pH 7.6), 1 mM MnCl2 and 1 mM MgCl2. The homogenate was then filtered through four layers of gauze and centrifuged for 10 min at 2,000 × g at 4°C. The precipitate was suspended in grinding solution and intact chloroplasts isolated by centrifugation for 5 min at 4,500 × g with a discontinuous gradient of 40% (v/v) and 70% (v/v) Percoll in a solution containing 330 mM sorbitol, 5 mM sodium ascorbate, 50 mM HEPES-KOH (pH 7.6) and 1 mM MgCl2. Intact chloroplasts were washed twice by centrifugation with wash buffer containing 330 mM sorbitol, 5 mM sodium ascorbate, 50 mM HEPES-KOH (pH 7.6) and 1 mM MgCl2. The intact chloroplasts obtained were resuspended in the wash buffer at a Chl concentration of 0.5 mg ml−1 and stored at 0°C.
Preparation of enzymes and antibodies
CF1 was isolated from broken chloroplasts of spinach based on the method described by Hisabori et al. (1993) with minor modifications. The broken chloroplasts were prepared by the standard procedure and washed five times with 10 mM sodium pyrophosphate to remove Rubisco. The chloroplasts were collected by centrifugation, suspended in 300 mM sucrose and 2 mM Tricine-NaOH (pH 8.0), and incubated for 10 min at 25°C. After centrifugation for 10 min at 15,000 × g at 4°C, the supernatant containing CF1 was obtained and displayed a weak yellowish color. The purity of the resulted CF1 fraction was normally >90%, estimated by Coomassie Brilliant Blue (CBB) staining after SDS–PAGE (Supplementary Fig. S1), and sufficient to observe the redox states of the γ subunit by Western blotting (Hisabori et al. 1993).
For preparation of the specific antibody against the regulatory region of the γ subunit, the partial polypeptide of the CF1 γ subunit comprising Ser163–Gln249 was expressed in Escherichia coli. The expressed polypeptide was obtained from the cells by a French pressure cell (5501-M, Ohtake Works) at 4°C, and purified by successive chromatography with a QAE Toyopearl column (Tosoh) with a 0–300 mM linear gradient of NaCl in 20 mM Tris–HCl (pH 8.0), 2 mM EDTA and 5 mM DTT, and an FPLC system with a Hi-Load 75PG column (GE Healthcare) eluted with 20 mM Tris–HCl (pH 8.0), 1 mM EDTA, 200 mM NaCl and 2 mM DTT. The purified polypeptide sample was then used as antigen to prepare the specific antibody.
The recombinant FBPase of A. thaliana chloroplasts lacking the signal sequence (Met59–Ala417) was expressed in E. coli. The cells were collected by centrifugation and disrupted by sonication. The supernatant resulting from centrifugation at 125,000 × g for 30 min at 4°C was applied to a DEAE-Toyopearl column (Tosoh) and eluted with a 0–300 mM linear gradient of NaCl in 25 mM Tris–HCl (pH 7.5). The peak fraction was collected and applied to a butyl-Toyopearl column (Tosoh). The protein sample was eluted with a 30–0% reverse gradient of ammonium sulfate and the peak fraction was stored. The purified FBPase protein was used as the antigen to prepare the antibody. Recombinant Trx-f was expressed in E. coli and purified as described previously (Stumpp et al. 1999).
AMS labeling of the γ subunit of CF1
The redox state of the γ subunit was determined by the standard procedure reported in Kobayashi et al. (1997) and Motohashi et al. (2001) using the specific thiol-labeling reagent AMS, which only labels the free thiol groups of cysteine residues. For complete oxidation or reduction, CF1 in 300 mM sucrose and 2 mM Tricine-NaOH (pH 8.0) was incubated for 90 min at 25°C with 50 μM diamide or 20 mM DTT and 19 μM Trx-f. After incubation, CF1 was precipitated by the addition of the ice-cold 10% (v/v) trichloroacetic acid and collected by centrifugation for 10 min at 10,000 × g at 4°C. The precipitate was washed twice with ice-cold acetone and then suspended into 4 mM AMS, 125 mM Tris–HCl (pH 7.5) and 1% (w/v) SDS for thiol modification. The solution was incubated for 1 h at 25°C and the proteins in the solution separated by SDS–PAGE using a 12% (w/v) polyacrylamide gel. The γ subunit was visualized using a specific antibody against γ and the redox level was determined from their mobility on the gel as follows. For the calculation of the reduced/oxidized proteins, the sample size loaded on the gel was controlled to avoid saturation of the signal after Western blotting.
Determination of the reduction/oxidation level of thiol enzymes
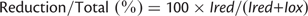
Photosynthetic reduction of thiol enzymes in intact chloroplasts
Freshly prepared intact chloroplasts (0.2 mg chl ml−1) were incubated for 10 min at 25°C in the dark, and illuminated for 10 min at 25°C with various light intensities. To avoid the change in temperature of the sample solution by illumination, a 5 cm water filter was placed between the lamp and the sample. After turning off the light, the chloroplasts were incubated in the dark. During this dark/light/dark transition, small aliquots (50 μl) were collected from the chloroplast solution at appropriate time intervals and directly mixed with the thiol group labeling solution containing 4 mM AMS, 125 mM Tris–HCl (pH 7.5) and 1% (w/v) SDS. The mixture was incubated for 60 min at 25°C. After centrifugation for 10 min at 10,000 × g at 25°C, supernatant proteins were separated by SDS–PAGE, the redox states of the γ subunit of CF1 and FBPase were visualized using specific antibodies and the band intensity was quantified as described.
Photosynthetic activation of the ATP hydrolysis activity of the chloroplast ATP synthase in intact chloroplasts
The freshly prepared intact chloroplasts (70 μg chl ml−1) were incubated in the same dark/light/dark conditions as those for the experiments for evaluation of the redox level of the γ subunit. During incubation, small aliquots (20 μl) were collected with an appropriate time interval and directly added to 180 μl of ATPase assay mixture containing 2 mM ATP, 50 mM Tricine-NaOH (pH 8.0) and 0.5 mM MgCl2 at 37°C. The mixture was incubated for 30 min and terminated by the addition of 2.4% (v/v) perchloric acid. Liberated phosphate was measured by a colorimetric method after formation of the Mo-phosphate coordinate (Technicon Corp. 1966).
Concentration of ATP in chloroplasts
At the time indicated, 50 μl of chloroplast aliquots were extracted and added to 10 μl of 12% (w/v) perchloric acid. Then 50 μl of the supernatant were neutralized with 125 μl of 2 M Tris-acetate (pH 7.7), and ATP was quantified by a luciferin–luciferase assay using an ATP bioluminescence assay kit CLS ΙΙ (Roche Diagnostics).
Photoreduction of the thiol enzymes in field-grown spinach leaves
Photosynthetic reduction of thiol enzymes in field-grown spinach leaves was determined using specific AMS labeling of thiol groups. To avoid the influence of artificial light particularly in the dark, the spinach planter was placed on the roof of an isolated building on campus. At the time indicated in Fig. 5, a spinach leaf specimen was collected from the plants and placed directly in liquid nitrogen to fix the reduced or oxidized status of the thiol enzymes in the cells. The frozen leaf was directly ground in liquid nitrogen and the powder obtained was placed into the thiol group labeling solution containing 4 mM AMS, 125 mM Tris–HCl (pH 7.5) and 10 mM EDTA, with 8 M urea to monitor FBPase and without urea to monitor CF1 γ. The sample was incubated for 30 min at 25°C to complete the labeling of thiol groups with AMS. The hydrophilic protein fraction was then isolated by addition of an equal volume of chloroform to the sample and the aqueous portion was collected after centrifugation. All proteins in the sample solution were then precipitated by 10% trichloroacetic acid. After washing twice with cold acetone, 125 mM Tris–HCl (pH 7.5) and 1% SDS were added to solubilize precipitates. The redox levels of γ and FBPase were visualized by Western blotting after SDS–PAGE using a 12% (w/v) polyacrylamide gel.
Funding
This work was supported by the Japan Science and Technology Agencey (JST) [ATP synthesis regulation project, ICORP].
Acknowledgments
We thank K. Yoshida and Y. Sugano for helpful discussion and valuable suggestions, and N. Tanaka for technical assistance.
Abbreviations
- AMS
4-acetoamido-4′-maleimidylstilbene-2,2′-disulfonate
- CFoCF1
chloroplast ATP synthase
- DBMIB
2,5-dibromo-3-isopropyl-6-methyl-p-benzoquinone
- DTT
dithiothreitol
- F1
coupling factor 1
- FBPase
fructose 1,6-bisphosphatase
- FCCP
carbonyl cyanide p-(trifluoro-methoxy) phenylhydrazone
- Trx
thioredoxin
- ΔμH+
the electrochemical gradient of a proton.